The two previous posts discussed the highly intelligent innovations of the individual cell to avoid microbe attacks; and the equally intelligent innovations of microbe invaders. The ubiquitin and SUMO systems, which tag and modulate proteins is a major target of both cell defenses and microbe attacks. Ubiquitin tags direct the protein to where and how it will operate in the cell. It is the file system for the protein shapes and functions. Both the cell and the virus produce many similar proteins and the ubiquitin and SUMO systems are the first line of attack between intelligent microbe invaders and the intelligent responses of the cells.
Ubiquitin is Ubiquitous
 Ubiquitin is a small protein of 76 amino acids that exists in most eukaryote cells with human and yeast versions 96% the same. There is no ubiquitin in prokaryotes.
Ubiquitin is a small protein of 76 amino acids that exists in most eukaryote cells with human and yeast versions 96% the same. There is no ubiquitin in prokaryotes.
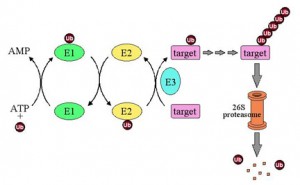
Ubiquitin’s end is a glycine amino acid, which attaches to a lysine of another protein. The process whereby it attaches is not simple, and involves three levels of enzymes. The first enzyme, E1, using an ATP energy particle to activate the process, transfers the ubiquitin to a second enzyme, E2, and then finally E3 attaches it to the protein that is being tagged. This very active complex process uses dozens of 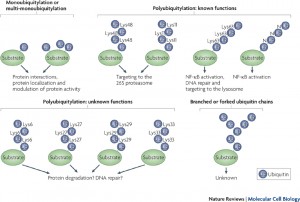 different E2 and hundreds of different E3 enzymes, producing a very large amount of different tags for a very wide range of different purposes.
different E2 and hundreds of different E3 enzymes, producing a very large amount of different tags for a very wide range of different purposes.
More than one ubiquitin can be added to a tagged protein, creating long branching chains each with a different purpose. Also, each of the 7 different lysines in the ubiquitin molecule can be used for these long chains. In this way an almost endless array of different tags are created,  for the vast amount of different protein functions in the cell.
for the vast amount of different protein functions in the cell.
The first major discovery of ubiquitin was in the destruction pathway, where it tags microbes, refuse and impaired organelles to be sent for disassembly. But, in fact it is an extremely critical technique of sorting and regulating most of the important cellular properties:
- Immune response
- DNA transcription
- Repair of DNA
- Production of antigens
- Birth of the organelles inside the cell
- Cell reproduction
- Development of the neural networks
- Degeneration of neurons and muscle cells
- Response to stress
- Regulation and modulation of receptors, ion channels
- Regulation of signaling pathways
- response to virus and bacteria invasion
- Manufacture of ribosomes
Ubiquitin is now shown to be very important in brain disorders including tagging of the amyloid precursor protein and fibrillary tangles in Alzheimer’s, Lewey body in Parkinson’s, Pick bodies in Pick’s disease and protein accumulations in Huntington’s disease
Recently, other related systems have been discovered called “ubiquitin-like proteins,” which also use the E1-E2-E3 system.
SUMO or Small Ubiquitin-like Modifiers
 Among the “ubiquitin-like proteins” SUMO is the most important. “Small ubiquitin-like modifier”, or SUMO, is a family of proteins that alter the amino acid products of messenger RNA and the Ribosome’s activity. It is a vast sister system to ubiquitin that doesn’t tag but attaches to proteins altering and regulating them, often using some of the same sequences as ubiquitin. When SUMO is attaches to a protein it is called SUMOylation.
Among the “ubiquitin-like proteins” SUMO is the most important. “Small ubiquitin-like modifier”, or SUMO, is a family of proteins that alter the amino acid products of messenger RNA and the Ribosome’s activity. It is a vast sister system to ubiquitin that doesn’t tag but attaches to proteins altering and regulating them, often using some of the same sequences as ubiquitin. When SUMO is attaches to a protein it is called SUMOylation.
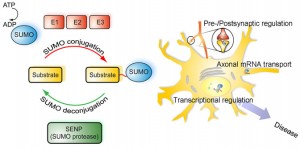 SUMO is critical in transport of molecules between the nucleus and the cytoplasm of the cell; the regulation of DNA transcription into RNA code; the apoptosis process; and the stabilization of proteins. It is vital in the cell’s response to stress and critical in the cell’s reproduction. SUMO operates on the signaling and enzyme cascades altering many of the proteins by sumoylation. It is present in all eukaryote cells, is very similar in all cells from yeast to humans, but, in vertebrates there are three versions, SUMO-1, 2 and 3.
SUMO is critical in transport of molecules between the nucleus and the cytoplasm of the cell; the regulation of DNA transcription into RNA code; the apoptosis process; and the stabilization of proteins. It is vital in the cell’s response to stress and critical in the cell’s reproduction. SUMO operates on the signaling and enzyme cascades altering many of the proteins by sumoylation. It is present in all eukaryote cells, is very similar in all cells from yeast to humans, but, in vertebrates there are three versions, SUMO-1, 2 and 3.
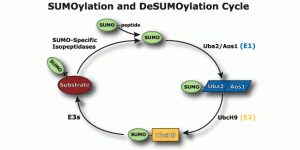 SUMO influences cellular activity, the use of cellular compartments, localization of processes, the stability of proteins and the molecules they are interacting with. It is now known to be critical in regulation of transcription, maintaining the genome, DNA repair, subcellular localization, nuclear transport, signaling, and creation of tumors. It appears to be as ubiquitous as ubiquitin.
SUMO influences cellular activity, the use of cellular compartments, localization of processes, the stability of proteins and the molecules they are interacting with. It is now known to be critical in regulation of transcription, maintaining the genome, DNA repair, subcellular localization, nuclear transport, signaling, and creation of tumors. It appears to be as ubiquitous as ubiquitin.
The Cell Versus the Viruses
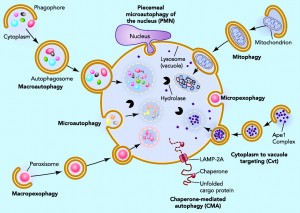 Individual cells tag microbes or their material with ubiquitin, which triggers engulfment of the microbe by vesicles. These vesicles are routed to the powerful lysosome and autophagy destruction processes in the cell. Cells are able to use different cellular compartments to wall off the virus with destruction of the contents of the compartment, or elimination of material from the compartment that the virus needs to replicate.
Individual cells tag microbes or their material with ubiquitin, which triggers engulfment of the microbe by vesicles. These vesicles are routed to the powerful lysosome and autophagy destruction processes in the cell. Cells are able to use different cellular compartments to wall off the virus with destruction of the contents of the compartment, or elimination of material from the compartment that the virus needs to replicate.
 Viruses, on the other hand, must both hijack cellular reproduction and must avoid the complex mechanisms the cell uses to kill the virus. The virus has to evade the innate and adaptive immune systems, and then the cell’s specific intrinsic immune system, often devised for the particular virus.
Viruses, on the other hand, must both hijack cellular reproduction and must avoid the complex mechanisms the cell uses to kill the virus. The virus has to evade the innate and adaptive immune systems, and then the cell’s specific intrinsic immune system, often devised for the particular virus.
Attacking and Defending the Subnuclear Structure
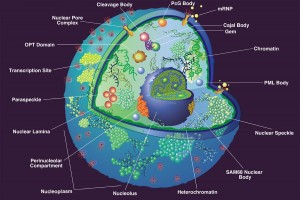 The battle between viruses and the host cell occurs everywhere in the cell but especially in the nucleus and at subnuclear structures.
The battle between viruses and the host cell occurs everywhere in the cell but especially in the nucleus and at subnuclear structures.
The nucleus is surrounded by a double membrane. In the nucleus there are many small bodies and compartments. One special subnuclear structure is called PML and appears to be related to the wide range of processes just mentioned and all appear to be highly regulated by sumolyation. This subnuclear structure involves hundreds of different proteins, all of which depend upon the SUMO instructions and alterations. The PML is a prime battlefield of the cell and the virus with both sides sending different SUMO and ubiquitin messages fighting for control of the cell.
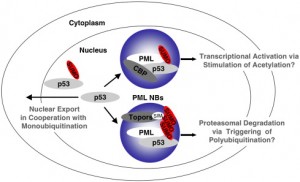 This PML is attacked by many viruses: DNA viruses including adenovirus, herpes, Ebstein Barr virus and apapillaomavirus; and RNA viruses including SARS, Ebola, HIV, entero virus, Dengue, corona, and picorna. This battle occurs also with a wide variety of extra and intracellular bacteria.
This PML is attacked by many viruses: DNA viruses including adenovirus, herpes, Ebstein Barr virus and apapillaomavirus; and RNA viruses including SARS, Ebola, HIV, entero virus, Dengue, corona, and picorna. This battle occurs also with a wide variety of extra and intracellular bacteria.
In each case a different set of factors are sent back and forth in the battle for the critical protein cascades that are the lifeblood of the cell.
The Cell SUMOs the Virus
Many of the viral proteins are sumoylated by the cell in a reversible way. Both DNA and RNA viruses that reproduce in the cytoplasm and in the nucleus are sumoylated. However, the virus can fight back by nullifying the effect of the SUMO by its own counteracting SUMO. The multiple ways that this process occurs can be either to the advantage of the virus or the invaded cell.
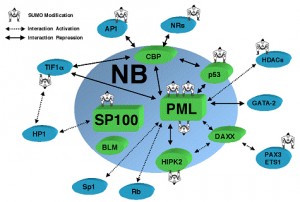 One example is the human cytomegalovirus, or HCMV, in the herpes family. The IE1 protein is critical for the virus to reproduce and to destroy the host cell. This is accomplished by targeting the PML in a process which involves at least fifty proteins that form a complex link to the histone chaperone molecules that protect the DNA. In this process, P53, a critical factor in stem cells, cell death and cancer, is altered with different SUMO markings by the virus and also by the cell. The sumolyation of the 1E1 affects its ability to stimulate interferon response to the HCMV infection. Also, IE2 of HCMV is essential to produce viral genes inside the cell and needs to be sumoylated by the cell in order to properly function.
One example is the human cytomegalovirus, or HCMV, in the herpes family. The IE1 protein is critical for the virus to reproduce and to destroy the host cell. This is accomplished by targeting the PML in a process which involves at least fifty proteins that form a complex link to the histone chaperone molecules that protect the DNA. In this process, P53, a critical factor in stem cells, cell death and cancer, is altered with different SUMO markings by the virus and also by the cell. The sumolyation of the 1E1 affects its ability to stimulate interferon response to the HCMV infection. Also, IE2 of HCMV is essential to produce viral genes inside the cell and needs to be sumoylated by the cell in order to properly function.
 There are so many different ways that sumolyation works that it is difficult to tell which side is benefitting from it. But, the battle continues constantly through these actions.
There are so many different ways that sumolyation works that it is difficult to tell which side is benefitting from it. But, the battle continues constantly through these actions.
A series of proteins produced by Epstein-Barr virus, and Adenovirus, stimulate a latent virus to become active when sumoylated. The sumoylation appears to be critical in the delicate balance between viruses who stay for years in a latent state inside of a cell and those who are awakened to kill the cell.
Vaccinia virus, influenza A and enterovirus all have important sumoylation during infection. In these cases the effect of the sumoylation alter the viruses location, which may help or hurt the virus or the cell, but helps the virus reproduction.
What is clear form all of this is that sumoylation can help the virus or the cell in multiple complex ways.
Virus Fights Back Interacting with SUMO
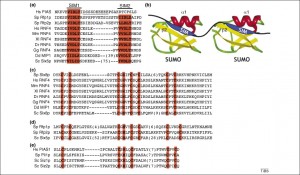 There are two distinct ways that SUMO attaches to proteins. One described above is a covalent chemical bond. The other called “SUMO interacting motifs” or SIMs. SIMs are noted to be important in DNA repair, transcriptional activation, nuclear body formation and protein turnover. It has recently been discovered that this is the way viruses often interact with SUMO. EBV has an enzyme with multiple SIM’s which increase their ability to kill host cells. SUMO appears to be critical for proteins to start the reproduction process for the virus.
There are two distinct ways that SUMO attaches to proteins. One described above is a covalent chemical bond. The other called “SUMO interacting motifs” or SIMs. SIMs are noted to be important in DNA repair, transcriptional activation, nuclear body formation and protein turnover. It has recently been discovered that this is the way viruses often interact with SUMO. EBV has an enzyme with multiple SIM’s which increase their ability to kill host cells. SUMO appears to be critical for proteins to start the reproduction process for the virus.
The alphaherpes family of viruses show that viruses can use SIM interactions with SUMO to counteract the effects of intrinsic immunity. Many viruses appear to have regions on their proteins that are highly related to SUMO enzymes. One example includes viruses with SUMO enzymes E3. In fact, this virus affects the very significant p53 by increasing a SUMO modification. One type of effect increases p53 activity and another decreases it.
Virus Stops SUMO and Evades Cells Defenses
There are many examples of viruses targeting the SUMO mechanism to take control of its activity, and therefore controlling a vast array of cellular behavior.
Intrinsic immunity of individual cells operates through proteins produced by the individual infected cells. The ubiquitin and SUMO pathways are utilized by the cells to attack the viruses, and likewise, the viruses utilize the same pathways to evade the defenses.
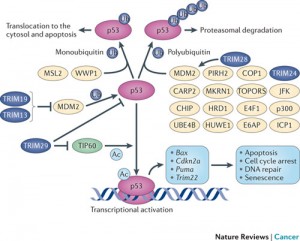 The TRIM family of proteins (tripartite motif) are an excellent example of proteins that provide intrinsic resistance for an individual cell and are manipulated by viruses. Several of these TRIM proteins have ubiquitin E3 activity (the third ubiquitin activating enzyme) and are used against the virus. TRIM19, which stops both DNA and RNA viruses from reproducing, accumulates near the virus DNA after it enters the nucleus. However, the virus can create an antagonist against the TRIMM. This is a battle between several different SUMO factors with the cell’s survival in the balance.
The TRIM family of proteins (tripartite motif) are an excellent example of proteins that provide intrinsic resistance for an individual cell and are manipulated by viruses. Several of these TRIM proteins have ubiquitin E3 activity (the third ubiquitin activating enzyme) and are used against the virus. TRIM19, which stops both DNA and RNA viruses from reproducing, accumulates near the virus DNA after it enters the nucleus. However, the virus can create an antagonist against the TRIMM. This is a battle between several different SUMO factors with the cell’s survival in the balance.
Another TRIM blocks HIV early binding to viral capsids which can alter the delivery of the HIV genes into the cell’s nucleus. This TRIM has multiple SUMO effects. In this case if SUMO1 is increased it helps the cell fight the HIV.
SUMO and Innate immunity – Which Side Controls Immune Systems
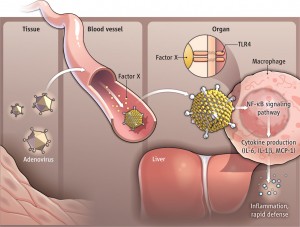 Innate immunity requires signals that send antiviral proteins in the regions surrounding uninfected cells, as well as inside of the infected cells. Pattern recognition receptors detect virus DNA and RNA and other virus proteins and trigger interferon. Sumoylation has major effects on production of interferon which activate helper immune cells.
Innate immunity requires signals that send antiviral proteins in the regions surrounding uninfected cells, as well as inside of the infected cells. Pattern recognition receptors detect virus DNA and RNA and other virus proteins and trigger interferon. Sumoylation has major effects on production of interferon which activate helper immune cells.
Given that SUMO has critical roles in modifying and regulating immunity it is easy to see the need for RNA and DNA viruses to hijack this process and suppress innate immunity signaling. In one example Ebola virus creates an antagonist forming a complex with multiple proteins to reduce DNA binding activity of cellular defenses. Also, Ebstein Barr virus has a similar mechanism. All of these are through sumolyation.
Language of Cells and Virus
 Previous posts have discussed how cells can self edit DNA code through RNA alternative splicing creating many different shaped proteins. Somehow the cell knows what the shape will be after splicing and after protein folding. But, even more complex is that ubiquitin knows how to tag these different shapes with specific codes of molecular chains in different locations. SUMO goes further and regulates these proteins with multiple tags.
Previous posts have discussed how cells can self edit DNA code through RNA alternative splicing creating many different shaped proteins. Somehow the cell knows what the shape will be after splicing and after protein folding. But, even more complex is that ubiquitin knows how to tag these different shapes with specific codes of molecular chains in different locations. SUMO goes further and regulates these proteins with multiple tags.
Both the cell and the virus innovate and make factors and proteins that constantly battle for control of the cell’s extremely complex processes. Both the virus and the cell use the tagging ubiquitin system and the regulating SUMO system to their own advantage. Innovations by both are constantly tipping the balance of power.
The many variations in the ubiquitin and SUMO pathways, and the unique ways that this coding can affect almost all cellular processes, makes the cascades a very important language that is helping and harming both sides. There are so many ways that SUMO can alter and ubiquitin can tag vital proteins, that both systems lend themselves to manipulation by the innovative intelligent invaders and the innovative intelligent cellular defender.
The use of the ubiquitin tagging and SUMO regulating systems is so elaborate and innovative, how we say that this is not an intelligent process? It appears that intelligent cells battle against intelligent invaders.