 Immune T cells have a very complex life, travelling to different regions of the body and maturing gradually through stages to be able to engage and fight a wide range of invaders. The T cell ( T for maturation in the thymus) is one of only two cells that edits and splices its own DNA to make a vast array of different receptors (the other cell is the immune B cell that makes antibodies). As remarkable as this process is, perhaps even more remarkable is the new data showing that immune T cells are critical for cognitive function. This function is through wireless communication with the brain (see Guest Blog, Scientific American – Wired and Wireless Brain).
Immune T cells have a very complex life, travelling to different regions of the body and maturing gradually through stages to be able to engage and fight a wide range of invaders. The T cell ( T for maturation in the thymus) is one of only two cells that edits and splices its own DNA to make a vast array of different receptors (the other cell is the immune B cell that makes antibodies). As remarkable as this process is, perhaps even more remarkable is the new data showing that immune T cells are critical for cognitive function. This function is through wireless communication with the brain (see Guest Blog, Scientific American – Wired and Wireless Brain).
Previous posts have begun to describe the enormous complexity of the immune wireless communication system—the cytokine signaling (as 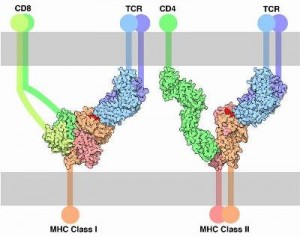 vast and as complex as the brain’s neurotransmitters) that uses thousands of signals for communication between immune and brain cells. Also, previous posts have described the production of the large complex MHC molecules (major histocompatibility complex) that sit on the surface of all of our cells giving information to the T cells about what lies inside the cell. Surprisingly, the MHC molecules, along with many other immune molecules, have dual function in fighting infection and in the normal function of the brain.
vast and as complex as the brain’s neurotransmitters) that uses thousands of signals for communication between immune and brain cells. Also, previous posts have described the production of the large complex MHC molecules (major histocompatibility complex) that sit on the surface of all of our cells giving information to the T cells about what lies inside the cell. Surprisingly, the MHC molecules, along with many other immune molecules, have dual function in fighting infection and in the normal function of the brain.
 But, now it appears that the T cell has entirely different capacities as well—helping learning and cognition by communicating with the brain through wireless communication. The T cell increases cognition under normal circumstances, and decreases cognition when there is infection and “sick” behavior.
But, now it appears that the T cell has entirely different capacities as well—helping learning and cognition by communicating with the brain through wireless communication. The T cell increases cognition under normal circumstances, and decreases cognition when there is infection and “sick” behavior.
There are actually many different kinds of T cells that all have extremely complex functions. The two most important and well known are CDC4+ and CDC8+. In order to make an extremely complex subject approachable, this post will introduce T cells by referring to them generally as “T cells” rather than the many subtypes, each with many different capacities and properties.
Immune Cells in the Brain
 The brain has been considered immune privileged (meaning the immune system largely avoids the brain in order to not cause trouble). This was considered dogma because there has not been evidence of large-scale immune activity other than the microglia cell, which is both glial and immune. Immune activity of microglia have been noted both in surveillance for infection and, also, in direct help with synapse maintenance and pruning (described in previous post).
The brain has been considered immune privileged (meaning the immune system largely avoids the brain in order to not cause trouble). This was considered dogma because there has not been evidence of large-scale immune activity other than the microglia cell, which is both glial and immune. Immune activity of microglia have been noted both in surveillance for infection and, also, in direct help with synapse maintenance and pruning (described in previous post).
It is extremely difficult to observe a small number of immune cells in the brain. It is, in fact, almost impossible to observe single cells among the vast networks of neurons, equally vast networks of astrocytes, constantly moving microglia and the very active complex extra cellular matrix. Now, it appears that T cells do enter the brain in small numbers and have critical communication and impact with normal brain function—not just during infections.
Surprisingly, recent research shows that if T cells are not in the cerebral spinal fluid (CSF), other white blood cells will cause inflammation. When T cells are present in the CSF, there is, instead, suppression of inflammation and positive communication to stimulate cognitive processes. For these functions, T cells use and respond not just to immune cytokines, but also to neurotransmitters serotonin, norepinephrine and dopamine.
Immune Cells in Brain Not Just For Infection
 Because the brain has multiple barriers to entry of blood cells, it was believed that any immune cells in the brain were due to illness. Or, that any immune cells in the brain would cause inflammation.
Because the brain has multiple barriers to entry of blood cells, it was believed that any immune cells in the brain were due to illness. Or, that any immune cells in the brain would cause inflammation.
There are many natural barriers to stop blood cells from entering the brain. The cerebrospinal fluid, CSF, is separate from the brain tissue. The blood brain barrier is another difficult obstruction to cross. Another important barrier is the very dense network of astrocytes just next to the blood vessels.
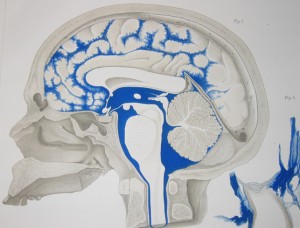 The cerebrospinal fluid, CSF, flows in the middle of the three membranes of the meninges—between the arachnoid membrane (attached to the dura and the skull) and the pia membrane covering the brain (the subarachnoid space).
The cerebrospinal fluid, CSF, flows in the middle of the three membranes of the meninges—between the arachnoid membrane (attached to the dura and the skull) and the pia membrane covering the brain (the subarachnoid space).
Despite previous dogma about “brain immune privilege” there are, actually, many immune cells in the CSF including B and T lymphocytes, macrophages, mast cells, and dendritic cells.
The T cell appears to be the leader of the immune cells in the CSF. T cells have a controlling effect on all other immune cells in the CSF. When T cells are present other cells are in check. Without T cells these cells become inflammatory and produce multiple cytokines that interfere with brain function. These specific cytokines, IL-1beta, IL-12 and TNF, have been previously described as producing sickness mental states, which interfere with learning and normal human behavior.
The Complex T Cell
The T cell passes through stages of development in the bone marrow, thymus and lymph tissues to become one of dozens of types of critical immune cells. Only 2% of T cells graduate from these stages to become a mature and activated T cell, that is, one that will rapidly multiply and fight disease in the body. The other 98% are killed because they don’t fulfill the exact qualifications needed to perform these difficult functions. There are a series of check points. If the immature T cells do not create the proper DNA edits they are rejected. At further stages, if they don’t create many very special complex receptors, they do not graduate from the check points and are eliminated.
A unique aspect of T cells is that they respond to specific pieces of molecules 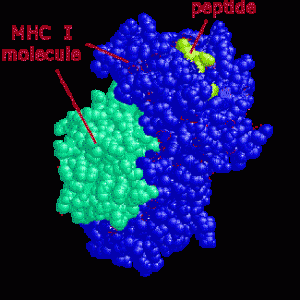 that are carried by large MHC molecules on the surface of other cells. This is equivalent to cells advertising their internal molecules on their surface. The advertised piece of protein (a peptide) is in the groove of the MHC (on the cell’s membrane) and is called an antigen. If the antigen is from a cancer cell, a damaged cell or has material from a microbe inside the T cell will attack and kill the cell (or direct other cells to do that).
that are carried by large MHC molecules on the surface of other cells. This is equivalent to cells advertising their internal molecules on their surface. The advertised piece of protein (a peptide) is in the groove of the MHC (on the cell’s membrane) and is called an antigen. If the antigen is from a cancer cell, a damaged cell or has material from a microbe inside the T cell will attack and kill the cell (or direct other cells to do that).
If the piece is from a normal cel, it is called a “self antigen.” Self antigens are very important because the T cell could use these to attack normal tissue. Therefore, most of these self antigens (but not all) are destroyed. If they are not destroyed then they would attack normal human cells causing autoimmune diseases such as multiple sclerosis. But, this is not the complete story.
The 98% of the T cells that are destroyed in the thymus and lymph tissues, are either cells that could not produce the complex machinery and receptors to graduate to the next phase of maturation, or a cell that is primed for a “self antigen” and is therefore dangerous to the body.
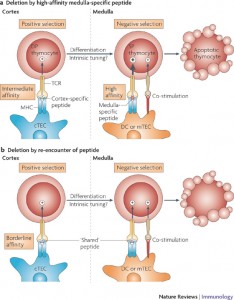 But, this process is much more complex than this simple narrative. In fact, it is the intensity of the attraction for self-antigens that allow T cells to graduate and live. If attraction is not strong enough then the cell is useless for future attack. If the attraction is too strong it will cause autoimmunity. And if it is “just right,” neither too strong nor too weak, it is allowed to continue on to the next level to become an active T cell. This process, which is not completely understood, will be discussed more in future posts dealing with T cell intelligence. After this selection process, the T cell roams the blood and lymph tissue browsing cells and searching for signs of invaders in the peptides that lie in the grooves of the MHC molecules on every cell.
But, this process is much more complex than this simple narrative. In fact, it is the intensity of the attraction for self-antigens that allow T cells to graduate and live. If attraction is not strong enough then the cell is useless for future attack. If the attraction is too strong it will cause autoimmunity. And if it is “just right,” neither too strong nor too weak, it is allowed to continue on to the next level to become an active T cell. This process, which is not completely understood, will be discussed more in future posts dealing with T cell intelligence. After this selection process, the T cell roams the blood and lymph tissue browsing cells and searching for signs of invaders in the peptides that lie in the grooves of the MHC molecules on every cell.
For the purpose of describing the cognitive effects of T cells it should be noted that if the T cell doesn’t have at least some affinity to brain tissue then the cognitive affects don’t occur. These effects are, seemingly, entirely separate from the normal microbe hunting effects and are turned off when the T cell is attacking.
T Cells for Brain Protection and Learning
 Damaged neurons, trigger more damage to neighboring neurons—called secondary degeneration. Animals without T and B lymphocytes have greater secondary neuron degeneration. If T cells are placed in animals with poor immunity, there is less secondary degeneration. Any T cell will help but if it is a T cell is responsive to antigens of the normal brain, the response is much greater.
Damaged neurons, trigger more damage to neighboring neurons—called secondary degeneration. Animals without T and B lymphocytes have greater secondary neuron degeneration. If T cells are placed in animals with poor immunity, there is less secondary degeneration. Any T cell will help but if it is a T cell is responsive to antigens of the normal brain, the response is much greater.
T cells, when they function in immunity, secrete cytokines that stimulate inflammation and other immune activity. For their cognitive functions, T cells secrete neurotrophins (molecules that help brain cells) that modulate astrocyte and microglia glutamate release. But, this only occurs if the T cell recognizes brain self-antigens to some extent.
T Cells – Stress and Learning
 Normal learning involves some stress response. Stress related to the subject of learning helps, other types do not. Chronic stress is very detrimental, impairing learning and decreasing the amount of new neurons used for learning.
Normal learning involves some stress response. Stress related to the subject of learning helps, other types do not. Chronic stress is very detrimental, impairing learning and decreasing the amount of new neurons used for learning.
Immune deficient mice with poor stress response don’t learn as well. T cells make this learning better.
The immune response is critical for the human stress response, for spatial learning and memory and for making new brain cells.
How Does This Work – T Cells in the CSF
The T cells effect on learning might be related to T cells invading the CNS in small undetectable amounts. More likely it is from direct effects on brain cells traveling in the CSF through cytokine communications.
Recently, it was found that the CSF normally has as many as 500,000 T cells. Performance of cognitive tasks is increased when T cells are in the CSF. When T cells were blocked in CSF, cognitive performance decreased. There is a lot of back and forth communication between T cells in the CSF and the brain.
When the brain is performing cognitive tasks, or has minor stress, there are signals sent to the cells in the immune system. Signals from the CSF are, also, sent to immune cells when there are pieces of microbes, or other brain molecules, such as pieces of myelin, debris, neurotransmitters and peptides.
The brain sends signals to the thymus, the lymph and bone marrow through the sympathetic and parasympathetic nerves. As well, cholinergic, epinephrine, norepinephrine and dopamine all signal to the immune cell receptors. It is not widely known that when neurotransmitters are secreted they diffuse through the tissue and are picked up by the immune cells.
T Cells Promote and Stop Inflammation
T cells can produce ACTH causing inflammation. When microbe patterns are picked up T cells become pro inflammatory and cognitive function is decreased.
T cells decrease inflammation through dopamine D1, D5 receptors in T cells and have an emergency brake by suppressing IL-12.
When learning is occurring, T cells secrete IL-4, which fights against any inflammation. Also, IL-4 stimulates more BNDF from astrocytes which is critical for growing new brain cells, learning and cognition.
Immune Surveillance of CNS by T Cells
Previously it was thought that antigens respond in the periphery to a microbe then send memory T cells to the brain. But, a more recent view is that T cells constantly monitor the CSF space where they meet antigen presenting cells.
The brain floats on CSF, which comes from arterial blood and a small amount from interstitial fluid. Cilia propel fluid to circulate in the ventricles through pores to spinal cord, brain stem, cerebellum and cortex. CSF is then absorbed to venous sinus blood. CSF drains along nerves, both cranial and spinal, to the lymph system.
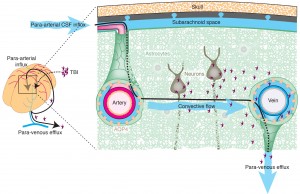 The brain doesn’t have a lymphatic system, but recently a glymphatic system has been described where waste is transported and processed (glymphatic means glia and lymphatic). (This week a very important discovery found that sleep induces increase in glymphatic cleaning of debris each night). The fluid between cells drains from channels around blood vessels into the CSF, where macrophages meet the antigens. They are also transported to the nasal mucosa then to the deep cervical lymph nodes (DCLN) in the neck.
The brain doesn’t have a lymphatic system, but recently a glymphatic system has been described where waste is transported and processed (glymphatic means glia and lymphatic). (This week a very important discovery found that sleep induces increase in glymphatic cleaning of debris each night). The fluid between cells drains from channels around blood vessels into the CSF, where macrophages meet the antigens. They are also transported to the nasal mucosa then to the deep cervical lymph nodes (DCLN) in the neck.
The glymphatic system works by CSF fluid mixing with interstitial fluid (ISF) and extracellular solutes including proteins, waste products, and excess extracellular fluid. Flow of material in the glymphatic system is facilitated by astrocytic aquaporin 4 water channels.
T Cells and Antigens in the CSF
Antigens are drained by this process from the brain into the lymph. Antigens from the CSF end up in the DCLN in the neck.
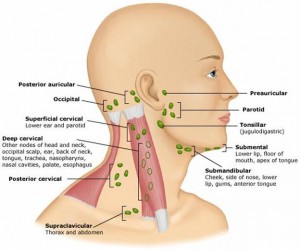 One of the easiest places for antigens to drain into lymph is in the nose, which is very close to the brain and CSF. This drainage into the lymph tissue occurs especially in olfactory roots to the nasal mucosa from frontal lobe to lymph of deep cervical lymph nodes. In an experiment if antigen is put into the CSF ventricle half goes to DCLN in 2 hours. Antigen antibody specific cells arrive in DCLN in four days.
One of the easiest places for antigens to drain into lymph is in the nose, which is very close to the brain and CSF. This drainage into the lymph tissue occurs especially in olfactory roots to the nasal mucosa from frontal lobe to lymph of deep cervical lymph nodes. In an experiment if antigen is put into the CSF ventricle half goes to DCLN in 2 hours. Antigen antibody specific cells arrive in DCLN in four days.
T cells enter the CSF and travel the entire region and then leave, going to DCLN. This is their route under normal brain circumstances. They travel this route directed by complex signals that make them adhere to different cells along the way. Special cytokines that direct travel are called chemokines. These chemokine directions are sent out and the T cells in their maturation have developed the appropriate receptors to receive and follow their direction.
When there is disease, T cells enter the brain from blood vessels during inflammation. Recently, it was shown that after infection memory T cells stay in the brain for future surveillance. Many T cells stay in brain after clearing of a virus.
Immune T Cells Are Critical For Cognitive Function
 T cells have the remarkable ability to self edit their own DNA, like antibody-producing B cells. They mature in the thymus through many stages, producing complex receptors that respond to the thousands of cytokine and neurotransmitter signals being sent throughout the body and brain. They also send out their own cytokines and neurotransmitters. They meet a specific receptor that matches a specific invader, and then shift gears and multiple rapidly to chase and fight the invader inside cells.
T cells have the remarkable ability to self edit their own DNA, like antibody-producing B cells. They mature in the thymus through many stages, producing complex receptors that respond to the thousands of cytokine and neurotransmitter signals being sent throughout the body and brain. They also send out their own cytokines and neurotransmitters. They meet a specific receptor that matches a specific invader, and then shift gears and multiple rapidly to chase and fight the invader inside cells.
But, they are much more complex. When not fighting invaders, they have a very unusual ability to help the brain function and are necessary for cognition. They, also, suppress inflammation while they help cognition. When they respond to microbes and increase inflammation they decrease cognition. This communication with the brain’s cognitive processes occurs through wireless signals while traveling in the CSF (and stopping other cells from causing inflammation). While all of the details are not completely understood, their presence in the cerebrospinal fluid is essential for communication that aids learning and brain health. Their absence leads to inflammation, illness and decreased cognition.
The T cell is part of the wireless brain, giving and receiving signals from a vantage point in the CSF. Future posts will discuss more about the remarkable intelligent T cell.