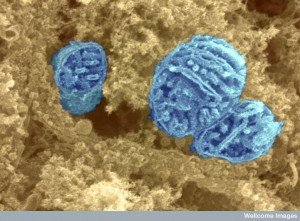
Mitochondria are essential energy producers for many of the key functions of the neuron, including the movement and recycling of the vesicles that carry neurotransmitters, the assembly and movement of the structural tubules, the generation of electric charge in axons and dendrites, and the maintenance of synaptic plasticity. They are needed throughout the neuron to provide fuel. It is not widely appreciated that they are, also, critical for the metabolism of amino acids, lipids, and steroids. They regulate the calcium levels in the cell which trigger the axon signal firing. They also produce free radicals and regulate the process known as apoptosis, whereby a cell is systematically dismantled without forming scars. Apoptosis, or programmed death, is the process that helps prune neurons trigged by microglia.
Mitochondria have extremely organized structures for responses to the neuron’s needs. Within the neuron, they must move to areas of high-energy activity. For example, large numbers are needed near the synapse and near the growth cones, the budding regions of the membrane that form new axons and dendrites in a neuron. When broken, some mitochondria travel back to the center of the cell to be dismantled; some are fixed by merging with outgoing healthy comrades. Currently, it is thought that several neurological diseases, including Huntington’s disease, Parkinson’s disease and Amyotrophic Lateral Sclerosis, known popularly as Lou Gehrig’s Disease, might be due to defective mitochondrial transport. There are many other diseases, including bipolar disorder, schizophrenia, and autism, for which there is, also, evidence of defective mitochondria functions.
Mitochondria Still A Microbe, But With Less Baggage
All microbes seek the easiest and lightest way to travel by shedding DNA and RNA whenever possible. They look to neighbors or symbiosis to pick up the functions, leaving them more mobile and effective in their chosen tasks. The mitochondria, an alpha-proteobacterium who joined forces with a larger cell two billion years ago to form our own eukaryote cells, has gradually shed DNA to the larger cell, in order to become an effective intracellular powerhouse.
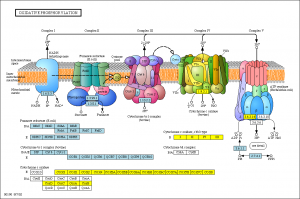 Along the membrane inside the mitochondria, the elaborate respiratory complex and the Krebs cycle transfer electrons to oxygen forming an electrochemical gradient that transfers protons to make most of the cell’s high energy particles. Mitochondria oxidize molecules capturing the bond energy, which is made available in the form of energy rich molecules ATP, GTP NADH2 and NADPH2. It also uses this gradient to buffer calcium signaling. When this gradient fails, the mitochondria is defective and signals prepare for its repair or destruction.
Along the membrane inside the mitochondria, the elaborate respiratory complex and the Krebs cycle transfer electrons to oxygen forming an electrochemical gradient that transfers protons to make most of the cell’s high energy particles. Mitochondria oxidize molecules capturing the bond energy, which is made available in the form of energy rich molecules ATP, GTP NADH2 and NADPH2. It also uses this gradient to buffer calcium signaling. When this gradient fails, the mitochondria is defective and signals prepare for its repair or destruction.
To perform these functions, mitochondria must stay in close communication with the larger cell and has had to develop new forms and capabilities to help with the very complex work of the human being.
Mitochondria DNA and Proteins
Inside our cells, mitochondria multiply by dividing in half similar to bacteria. Unlike bacteria they can also fuse with other comrades in the cell. In addition, unlike other bacteria, they have many copies of circular DNA, made inside the mitochondria at times when the mitochondria is not dividing.
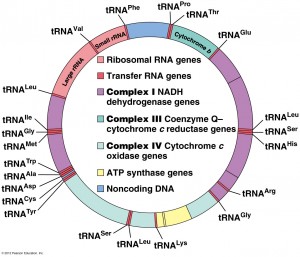 There are 100-10,000 double stranded separate copies of mitochondrial DNA in each neuron, each having 15,000-17,000 base pairs. One circular strand is guanine-rich called the heavy strand with 28 genes, and the other cytosine-rich called the light strand with 9 genes. Of the 37 genes, 13 are for respiratory proteins , 22 are for transfer RNA and two are for the small and large subunits of the mitochondrial ribosome. The DNA recombines but only in the one mitochondria that may or may not have effects generally.
There are 100-10,000 double stranded separate copies of mitochondrial DNA in each neuron, each having 15,000-17,000 base pairs. One circular strand is guanine-rich called the heavy strand with 28 genes, and the other cytosine-rich called the light strand with 9 genes. Of the 37 genes, 13 are for respiratory proteins , 22 are for transfer RNA and two are for the small and large subunits of the mitochondrial ribosome. The DNA recombines but only in the one mitochondria that may or may not have effects generally.
All other proteins used in the mitochondrial division and fusion are made in the neuron’s nucleus. Although only making 13 proteins themselves, mitochondria use more than 1,000 different proteins, which are different in different species and in different human tissues. Some of the DNA in mitochondria that are used to make RNA are originally from phage viruses; some are from other bacteria. Mitochondria still make their own transfer RNA and 2 types of complex ribosomes not from bacterial lineage—a mix of old and new DNA.
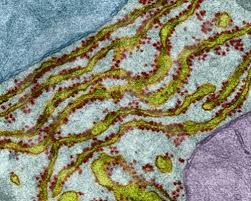 Most proteins used by the mitochondria are transported and assembled by machines in the neuron’s endoplasmic reticulum. Many complex pathways regulate the building blocks of mitochondria, stimulated by nutrients, environment and other factors. The machinery is triggered and coordinated by protein factors and RNA signals. Mutations of any part of this process can lead to many different significant mitochondrial diseases, including cancer and Alzheimer, Parkinson’s and Huntington’s.
Most proteins used by the mitochondria are transported and assembled by machines in the neuron’s endoplasmic reticulum. Many complex pathways regulate the building blocks of mitochondria, stimulated by nutrients, environment and other factors. The machinery is triggered and coordinated by protein factors and RNA signals. Mutations of any part of this process can lead to many different significant mitochondrial diseases, including cancer and Alzheimer, Parkinson’s and Huntington’s.
Mitochondria are critical in most functions of the cell and therefore error correction is critical. Contact between the neuron and the mitochondria involves communication about shared functions including calcium regulation, lipid manufacture and cellular autophagy.
Nucleoids are Packaged Mitochondria DNA
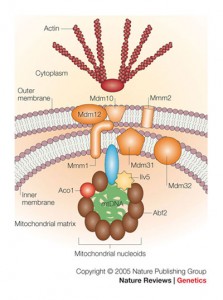 The DNA of the mitochondria are packaged tightly in a structure called the nucleoid. This is similar to human DNA being wound in spools called histones. Nucleoids are then distributed around the mitochondria for different functions. The mitochondrial chromosome can be shared among many individual mitochondria that are in the form of one large cell–a syncytium. With possibly thousands of nucleoids in one large cell, they respond to the “mitochondrial network,” which is constantly changing shape and function, while traveling between many different spaces in the huge neuron. The large fused mitochondrion can be a long, tubular and branched system inside the cell that is constantly changing due to fusion and division.
The DNA of the mitochondria are packaged tightly in a structure called the nucleoid. This is similar to human DNA being wound in spools called histones. Nucleoids are then distributed around the mitochondria for different functions. The mitochondrial chromosome can be shared among many individual mitochondria that are in the form of one large cell–a syncytium. With possibly thousands of nucleoids in one large cell, they respond to the “mitochondrial network,” which is constantly changing shape and function, while traveling between many different spaces in the huge neuron. The large fused mitochondrion can be a long, tubular and branched system inside the cell that is constantly changing due to fusion and division.
Nucleoids have a wide variety of organization and structure. In humans the nucleoids are more solitary. The male versions are destroyed after fertilization and only the female nucleoids in the egg are passed on to the fetus. In oocytes there is an elaborate structure called Balbiani body, including ER and Golgi, that is involved in the placement and copying of nucleoids. This process is not well understood currently. Ordinary cellular DNA duplicates in a highly structured manner only during cell division. This extremely complex process is directed by microtubule structures including the spindle. Mitochondria nucleoids duplicate at many different times, but only some of them. Without the elaborate scaffolding molecules of the cellular division, it is not clear why or how this happens. When a mutation occurs, it is only in one mitochondria and a very small proportion of the DNA present in the total mitochondria.
The Nucleoids are Distributed in the Mitochondria
A large protein complex, called the ERMD integrator (ERMD is short for endoplasmic reticulum associated mitochondrial division), distributes the nucleoids along the inside of the mitochondria in different configurations. This complex also signals to the host cell while monitoring mitochondrial behavior. There is constant communication for critical functions such as division, fusion of mitochondria and cell death.
 Mitochondrial division occurs along the microtubule transport routes to help provide the necessary energy in different regions of the neuron. The dynein motor is a major transporter of mitochondria along the microtubule transport tracks. Dynein and dynein related proteins, called DPR, are critical for microtubule distribution, multiplication and quality control.
Mitochondrial division occurs along the microtubule transport routes to help provide the necessary energy in different regions of the neuron. The dynein motor is a major transporter of mitochondria along the microtubule transport tracks. Dynein and dynein related proteins, called DPR, are critical for microtubule distribution, multiplication and quality control.
DPR is also involved in destruction of defective mitochondria, called mitophagy. In this process, DPR attracts Bcl-2 BAS and activates it on the outer membrane. This triggers permability of the mitochondrial membrane.
Much of the communication between the neuron and the mitochondria occurs at the endoplasmic reticulum, or ER. Specific defects in this communication between the ER and mitochondria occur in neurodegenerative diseases. Signaling proteins are involved in Huntington’s. Alzheimer’s has a altered ER-mitochondria contact.
Mitochondria Have Critical Relationship with the Neuron’s Endoplasmic Reticulum
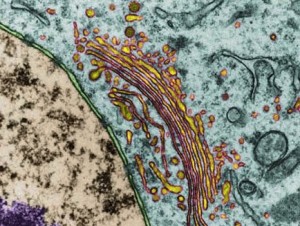 The endoplasmic reticulum, ER, is a series of interconnected membrane sacs and tubes, called cisternae, that are very close and continuous with the membrane of the cell’s nucleus. Rough ER have many ribosomes and are where proteins are manufactured after messenger RNA travels from the nucleus. The smooth ER are where lipids and carbohydrates are manufactured and incorporated into complex machinery with proteins.
The endoplasmic reticulum, ER, is a series of interconnected membrane sacs and tubes, called cisternae, that are very close and continuous with the membrane of the cell’s nucleus. Rough ER have many ribosomes and are where proteins are manufactured after messenger RNA travels from the nucleus. The smooth ER are where lipids and carbohydrates are manufactured and incorporated into complex machinery with proteins.
There are special parts of the ER that communicate with and regulate mitochondria, called mitochondria-associated ER membrane, or MAM. The juncture between the ER and specific mitochondria operates exchanges of lipids, proteins, calcium buffering, control of making new mitochondria and many other factors including specific communications about mitochondrial movement throughout the neuron.
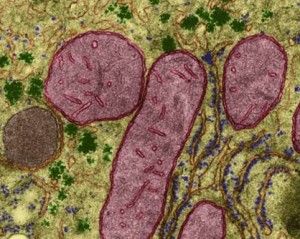 Some of the communication between ER and mitochondria occurs when the mitochondria is in close physical contact—they are tethered together at large sites called the tethering complex. Mitochondria are very dependent upon receiving lipids and proteins to make the complex internal membrane structures and this is accomplished at the tethering sites with the ER. Mitochondria then travel throughout the neuron with behavior regulated by signaling and the dynamic cycle of fusion and division.
Some of the communication between ER and mitochondria occurs when the mitochondria is in close physical contact—they are tethered together at large sites called the tethering complex. Mitochondria are very dependent upon receiving lipids and proteins to make the complex internal membrane structures and this is accomplished at the tethering sites with the ER. Mitochondria then travel throughout the neuron with behavior regulated by signaling and the dynamic cycle of fusion and division.
The mitochondria makes critical proteins in their smaller unique ribosomes attached to the internal membranes, but needs to import many phospholipids and protein-lipids. Most lipids are imported from the ER at the junction.
The ER is also involved in triggering cell death by apoptosis, a programmed destruction of the cell that leaves little trace or scar. It does this by altering the calcium balance in the mitochondria. The mitochondria normally are the calcium buffers for the neuron.
It has been found that stress from either the ER or the mitochondrion drastically affects the other. The mitochondria is linked to ER and together they regulate calcium. They allow calcium to propagate as a wave in local areas through the linking of the membranes of mitochondria and ER. They can either enhance metabolism or lead to cell death. Also, the ER connection can contribute to mitochondria division or fission.
Dynamic Control of Energy Needs and the Number of Mitochondria
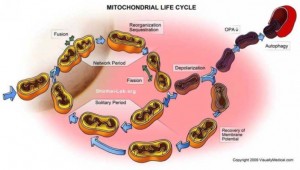 In communication with the neuron’s ER, the mitochondria move throughout the soma, axon and dendrites. They are constantly moving and changing shape with a dynamic process related to the needs of the particular space they are in and their own condition relative to their critical tasks.
In communication with the neuron’s ER, the mitochondria move throughout the soma, axon and dendrites. They are constantly moving and changing shape with a dynamic process related to the needs of the particular space they are in and their own condition relative to their critical tasks.
When mitochondria have spent their ability to maintain a gradient for transfer of electrons, they are either restored by the energy of other comrades through fusion, or they are moved to a process of destruction through division. The dance of producing more mitochondria and more energy is regulated through a complex system of quality control, from the communication of both ER and mitochondria, stimulating divisions and fusions. Both the individual mitochondrion and the large mitochondrial network of the entire cell is regulated in this process directed from the ER.
This dynamic includes dividing to make more mitochondria or to wall off a defective part and the opposite fusing with other mitochondria creating a larger network. The post next week will continue this complex story of fusion, division, quality control and cell death.
Intelligent Mitochondria Communication with Neurons
 Mitochondria respond instantly to mental processes and provide the energy that drives the neuron. While spawned near the cell body, mitochondria travel throughout the entire cell, including axons that can be two feet long, to provide energy at synapses. The neuron needs them for energy, buffering of the critical calcium signals, the movement of vesicles providing material to the far-reaching areas of the neuron, energy for transmission of neurotransmitters, scaffolding, neuroplasticity, the electrical spike along the axon, and the metabolism that builds proteins, lipids and steroids. They are part of the quality control system for the entire cell including programmed cell death. These functions are only part of the intelligent mitochondria communication with neurons.
Mitochondria respond instantly to mental processes and provide the energy that drives the neuron. While spawned near the cell body, mitochondria travel throughout the entire cell, including axons that can be two feet long, to provide energy at synapses. The neuron needs them for energy, buffering of the critical calcium signals, the movement of vesicles providing material to the far-reaching areas of the neuron, energy for transmission of neurotransmitters, scaffolding, neuroplasticity, the electrical spike along the axon, and the metabolism that builds proteins, lipids and steroids. They are part of the quality control system for the entire cell including programmed cell death. These functions are only part of the intelligent mitochondria communication with neurons.
This small microbe, evolving in the eukaryote cell for two billion years, communicates constantly with the much larger neuron. This intelligent communication determines the mitochondria’s behavior and the fate of the neuron and the brain. The next post will look further into this complex dynamic intelligent relationship.