 T cells have elaborate communication with almost all other cells. They are the master regulators of the immune system. But, they also converse with brain cells, blood vessel cells, and lining cells of the gut and skin. Previous posts have described communication with T cells and brain cells that are vital to keep normal cognition going and to alter it during infections with the “sick feeling” –produced by a signal from T cells. These posts noted that T cells are involved in the signals that stimulate production of new neurons in the hippocampus. as part of the memory system. When depression occurs, these neurons are diminished and stimulating new neurons correlates with treatments that improve depression.
T cells have elaborate communication with almost all other cells. They are the master regulators of the immune system. But, they also converse with brain cells, blood vessel cells, and lining cells of the gut and skin. Previous posts have described communication with T cells and brain cells that are vital to keep normal cognition going and to alter it during infections with the “sick feeling” –produced by a signal from T cells. These posts noted that T cells are involved in the signals that stimulate production of new neurons in the hippocampus. as part of the memory system. When depression occurs, these neurons are diminished and stimulating new neurons correlates with treatments that improve depression.
It is only recently that a much larger amount of activity of T cells in the brain has been found. A large variety of new types of cells have been found. Also, signaling has been found with other immune cells, such as the antigen presenting cells (APCs), but also other immune cells and glia cells. The new research is so fertile that the ramifications of all these new cells are just being discovered. There is much important material that is not yet understood. This post is an update of T cell complexity in the brain. There are many new types of immune cells found that interact with T cells both inside and outside of the brain causing effects in the brain. The current research is confusing but shows much more activity that will be eventually clarified.
 For a long time, it was thought that normally there were no immune cells in the brain. Then microglia were discovered as resident immune cells and then the existence of many T cells in the CSF. In recent years, the concept of “immune privilege” in the brain has been modified. Microglia were found to be in constant contact with T cells and other immune cells. But, it was also discovered that there is normally a wide range of T cells in the brain that converses actively with all brain cells, but also with all other immune cells. It is difficult to observe one small cell in the vast brain so it has been difficult to discover all the types of T cells in the brain. Now, new research is describing more T cell activity. Recently, lymph drainage of the brain has been described (see previous post on brain compartments and barriers).
For a long time, it was thought that normally there were no immune cells in the brain. Then microglia were discovered as resident immune cells and then the existence of many T cells in the CSF. In recent years, the concept of “immune privilege” in the brain has been modified. Microglia were found to be in constant contact with T cells and other immune cells. But, it was also discovered that there is normally a wide range of T cells in the brain that converses actively with all brain cells, but also with all other immune cells. It is difficult to observe one small cell in the vast brain so it has been difficult to discover all the types of T cells in the brain. Now, new research is describing more T cell activity. Recently, lymph drainage of the brain has been described (see previous post on brain compartments and barriers).
A Wide Range of New Types of T Cells in the Brain
 T cells in the brain prevent infections from microbes that have entered the brain, but can also produce damage through inflammation and autoimmunity. Recently, memory cells in the brain have been found, as well as CD4+, CD8+ and regulatory T cells.
T cells in the brain prevent infections from microbes that have entered the brain, but can also produce damage through inflammation and autoimmunity. Recently, memory cells in the brain have been found, as well as CD4+, CD8+ and regulatory T cells.
Special molecules, used throughout the body to advertise microbes and damage for T cells (MHC), are different in brain tissue. Also, it is difficult for immune cells to cross blood brain barrier and into CSF (see post on brain barriers and microbes crossing barriers). The lymph drainage is different than other organs changing how T cells can operate.
Unique T cells in Brain
 Many types of T cells have particular jobs in the periphery and somewhat different function in the brain.
Many types of T cells have particular jobs in the periphery and somewhat different function in the brain.
CD4+ T cells differentiate into multiple kinds of T helper cells in periphery with different receptors and chemokine signals and integrins. Integrins and chemokines call Th (T helper) cells to various tissues. These can cause different types of problems in the brain tissue in various regions. Inflammation calls for the special regulatory T cells to come to the brain—Forkhead box P3 (FOXP3) T cells to the brain. FOXP3 have been described in previous posts as stopping food allergies, but also help tamp down inflammation in the brain as well.
Other types of T cells stay in the brain for various purposes. CD8+ memory T cells (TRM) stay after virus infections. CD4+ also become resident after inflammation and stay.
There are many new findings and much is uncertain. Problems occur in the brain from viruses and several bacteria entering the brain. Another set of problems are autoimmune diseases. Antigens can be evaluated by T cells in lymph tissues from the brain and they can call for actions. Viruses could infect cells in the brain attracting T cells. Oligodendrocytes have self-antigens that T cells respond to as well. This could be part of Alzheimer’s and Parkinson’s with T cells in the CSF related to these diseases. But, these are not known to cause degenerative illness. MS does have self-antigens related to T cells.
 Triggers for T cell responses in the brain might not be from the viruses in the brain. Other mechanisms might be the cause of MS. MS related immune cells are not brain focused but adaptive cells.
Triggers for T cell responses in the brain might not be from the viruses in the brain. Other mechanisms might be the cause of MS. MS related immune cells are not brain focused but adaptive cells.
Lymph epithelial cells are not found in the brain tissue, but in peri-vascular compartments. Lymph nodes collected from dead oligodendrocytes had antigens against myelin. Dural sinuses drain as noted in previous posts. ISF drains into CSF via glymphatic system. CNS tissue could possibly drain in meninges lymph channels. Dura lymph has MHC II cells. It could be part of mechanism to eliminate T cells reacting incorrectly causing tolerance. CNS antigens drainage might cause tolerance not immunity. It might take microbe molecules to change this.
Cells that Present Molecules to T Cells in the Brain
 Reactivation of T cells in choroid, meninges, perivascular, and tissue and necessary for attack mode. Only class MHC 1 signals appear to occur in the tissue. Astrocytes and microglia can also interact with class II MHC in labs, but this hasn’t been proven live. MHC II on myeloid blood cells in meninges, perivascular can activate T cells for invasion with special cells CD11c+ from outise.
Reactivation of T cells in choroid, meninges, perivascular, and tissue and necessary for attack mode. Only class MHC 1 signals appear to occur in the tissue. Astrocytes and microglia can also interact with class II MHC in labs, but this hasn’t been proven live. MHC II on myeloid blood cells in meninges, perivascular can activate T cells for invasion with special cells CD11c+ from outise.
Cells that present molecules are called APCs or antigen presenting cells. The most common cells that present material to be evaluated by T cells are dendritic cells (DCs). Another type are macrophages. There are now many different DCs and macrophages found in the brain with different purposes interacting with T cells.
In meninges and choroid, DCs are different than microglia and monocytes, using CD8a+ some of the time. DCs last a week and more and come from bone stem cells—pre-DCs. With inflammation, monocytes and macrophage types have other receptors and present to CD4+T cells and CD8+. These are like DCs from monocytes.
T cells are re-stimulated by macrophages in the peri-vascular regions that are similar in lineage to microglia. This a unique line. Another line of macrophages mixes with monocytes in the blood. Macrophages in perivascular region are more like microglia than other macrophages near choroid cells. Macrophages in the meninges interact with new T cell travelers. Integrin helps connect them.
B cells Interacting with T cells in the Brain
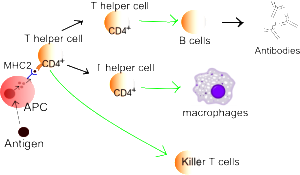 B cells can also perform as APC cells related to their particular antigen with MHC II. Few B cells are in CSF, but some are in the tissue. They specialize in activating Th1 cells rather than Th17 cells to produce inflammation. In chronic inflammation, there were B cells in meninges and CSF. They cause inflammation in limited compartments.
B cells can also perform as APC cells related to their particular antigen with MHC II. Few B cells are in CSF, but some are in the tissue. They specialize in activating Th1 cells rather than Th17 cells to produce inflammation. In chronic inflammation, there were B cells in meninges and CSF. They cause inflammation in limited compartments.
The latest research is complex and specific APCs help determine unique immune responses in various compartments.
Viruses in the Brain Trigger T Cells in Multiple Ways
 Some viruses cross vascular lining cells to enter the brain, such as West Nile. Some enter through immune cells travel into the brain, such as HIV often in monocytes. Another JCV virus uses B cells for entry. Some viruses enter neurons in the periphery and travel backward (herpes, polio, rabies). Antigens from these can come from lymph tissues in the periphery or in the brain draining to neck nodes.
Some viruses cross vascular lining cells to enter the brain, such as West Nile. Some enter through immune cells travel into the brain, such as HIV often in monocytes. Another JCV virus uses B cells for entry. Some viruses enter neurons in the periphery and travel backward (herpes, polio, rabies). Antigens from these can come from lymph tissues in the periphery or in the brain draining to neck nodes.
Most often T cells are right at the drainage site that respond. Special types of T cells are activated right there to enter the brain. Signals include cytokines and chemokines. Integrins are signals used for travel into the meninges and brain tissues. Special signals are needed, often with complex sequences.
For example, T cells for a particular antigen can be attracted to the perivascular region (CXCR4) then they are stopped there until it is altered to a CXCL12 signal. Different signals are used by T cells responding to self-antigens and these virus associated antigens. Many different complex combinations of signals are needed for each virus (CXCL10 to CXCR3 and others). Some of these signals can work in opposite ways with different viruses. Some viruses are controlled in the perivascular region. Others need full invasion and can cause brain inflammation damage and autoimmune damage.
Different T cell types also have different properties in these different situations. For example, Th1 is usually opposite to Th17, but both can work to stop virus CNS infections.
T cell Autoimmunity
 The types of microbes that accumulate on lining cells, most prominently in the gut, but also elsewhere, are very vital to the types of immune reactions that occur based on self-antigens. The gut cells stimulate particular type of Th17 helper cells. These reactions come from APC cells in the lymph tissues that lie in the gut.
The types of microbes that accumulate on lining cells, most prominently in the gut, but also elsewhere, are very vital to the types of immune reactions that occur based on self-antigens. The gut cells stimulate particular type of Th17 helper cells. These reactions come from APC cells in the lymph tissues that lie in the gut.
Another possibility is that particles from the gut travel throughout the body where responses occur. These responses to the gut microbe particles trigger APCs to activate T cells to become Th17 helper cells. This response occurs in the lymph tissue. These metabolites and particles from gut microbes have influences on astrocytes and microglia as well.
T Helper Cells -Th1 and Th17 in the Brain
 CD4+ T cells transform into multiple sets of helper cells with very different behavior. Th1 and Th2 work with cytokines like IL-4 to fight viruses and parasites. Th1 is closely related to autoimmune responses in the brain such as multiple sclerosis.
CD4+ T cells transform into multiple sets of helper cells with very different behavior. Th1 and Th2 work with cytokines like IL-4 to fight viruses and parasites. Th1 is closely related to autoimmune responses in the brain such as multiple sclerosis.
Another helper cell Th17 produces autoimmune inflammation in the brain (EAE or experimental autoimmune encephalitis. Th17 T cells produce cytokines IL-17A,F, IL-21 and IL-22 related to response to bacteria and fungi.
One of the unique T cells is a CD8+ MHC I type in the gut lamina propria region. They are produced in response to gut metabolites. These T cells go to the liver and are stimulated by signals from IL-12 and IL-18 to secrete interferon- g. These are found in the brain as well, especially when multiple sclerosis recurs. They travel from outside the brain using integrin adherence signals. Their exact function is not yet clear and they may actually be helping by stimulating b cells or not.
 There are a group of Th helper cells that are stimulated by gut microbe products that then travel to other organs and might be part of the trouble and the solutions.
There are a group of Th helper cells that are stimulated by gut microbe products that then travel to other organs and might be part of the trouble and the solutions.
When a T cell happens to be in the brain and then is activated it is a rare event most often related to inflammation in the brain. There are a lot of questions as to how these T cells get there. Normally there are few DCs and macrophages in the meninges, choroid, and spaces around blood vessels. But, when there is inflammation, there are APCs that can trigger T cells in these brain spaces. These activated T cells are then able to enter brain tissue.
T Helper Cells in Brain Inflammation
 Both Th1 and Th17 can cause autoimmunity inflammation responses in the brain. Both can cause inflammation in experimental conditions. Different types of integrin adherence and guidance molecules of particular types are involved. Also they entered in specific brain regions—Th17 brainstem and cerebellum and Th1 in the spinal cord. There are other adhesion molecules that help this travel and entry.
Both Th1 and Th17 can cause autoimmunity inflammation responses in the brain. Both can cause inflammation in experimental conditions. Different types of integrin adherence and guidance molecules of particular types are involved. Also they entered in specific brain regions—Th17 brainstem and cerebellum and Th1 in the spinal cord. There are other adhesion molecules that help this travel and entry.
Th17 produce damage that was once thought to be from Th1 cells. There is overlap in their use of cytokines GM-CSF. These signals are so complex that it is possible that there are multiple other subsets with different characteristics.
IL-23 is involved in the damage that Th17 will do. The specific damage appears to have many variables including the particular microbes and their signals. The signal IL-23 is not enough to cause the damage, but rather it is part of specific circumstances that vary where damage can occur.
 Molecular pathways that contribute to T cell types that contribute to inflammation include many that regulate how Th17 turns out. Multiple different factors affect the genetics of Th17. FOXP3 T cells have many different pathways of specialization.
Molecular pathways that contribute to T cell types that contribute to inflammation include many that regulate how Th17 turns out. Multiple different factors affect the genetics of Th17. FOXP3 T cells have many different pathways of specialization.
Th17 is triggered in a variety of different ways. Three in particular trigger specific genetic networks that produce the special T cell RORγt including the signals BATF, IFN regulatory factor 4 (IRF4) and STAT3. But there are a variety of other factors that alter one of these three producing other subsets of Th19. The three factors make room for RORγt by establishing especial three dimensional structures of chromatin. Some of these pathways alter the extent of producing inflammation.
Another pathway produces IL-17 and Th17 cells and brain inflammation of EAE.
New micro RNAs have been found to be signals that alter production of factors that regulate Th17 and thus inhibit production of these T cells. Another set increase the production of Th17. One vital pathway for production is the well-known PI3K-AKT pathway. Multiple tags on RNA have indirect methods of inhibition.
Altering T Cell Behavior While They Are in the Brain
 Even the cell Th17 can go both ways to inhibit or produce inflammation. In fact, even cells that were once Th17 can go both ways. IL-23 signals makes them destructive and the signal IL-10 the other way to resolution of inflammation in brain and gut.
Even the cell Th17 can go both ways to inhibit or produce inflammation. In fact, even cells that were once Th17 can go both ways. IL-23 signals makes them destructive and the signal IL-10 the other way to resolution of inflammation in brain and gut.
FOXP3 T cells are very involved in protecting against food allergies in the gut, (see post), but they have plasticity and they can produce IL-10 when there is great inflammation and tamp it down, which is what they do for all the cells that might attack food particles. This is a re programming of T cells that can occur with many T cells. Th1 can have many different effects as well. So much so there is a question as to whether it really is a separate species or a stage that many T cells can engage in.
A conclusion of these studies is that a wide variety of cell subtypes can produce IL-17 and a different group IL-10 with opposite effects on other cells. All of this occurs through genetic networks triggered by signals and pathways.
The list of factors that trigger these effects is growing including stimuli from the environment, metabolic signals, many cytokines, the strength and variations of T cell receptors, co stimulation signals (like integrin), and others.
CD8+ T cells in Brain Inflammation
 These cells are mostly cells of action, not regulation of other cells and they kill viruses in several different ways. It had been thought that they also are related to autoimmune attacks in MS responding to an antigen, which is unknown, possibly from viruses, unrelated to MS. They do produce the IL-17 cytokine, which has been implicated in damage.
These cells are mostly cells of action, not regulation of other cells and they kill viruses in several different ways. It had been thought that they also are related to autoimmune attacks in MS responding to an antigen, which is unknown, possibly from viruses, unrelated to MS. They do produce the IL-17 cytokine, which has been implicated in damage.
Another CD8+ cells is for remote memory Trm. These are vital for stopping re-infection. They hang out in tissues waiting for a re-infection. They are different from the usual memory T cells that travel in the blood and respond. These Trm cells have a particular kind of integrin that allows them to adhere and stay in the correct tissue and they have other receptors that keep them there as well (CD69). These are necessary to keep them in the brain at the right places. They have pathways that inhibit their usual tendency to travel. They appear to need IL-15 for survival there. These studies have found that there needs to be very particular signals that maintain these special memory T cells in particular tissues, different in each tissue, such as near blood vessels or near the surface.
What is remarkable about these Trm memory cells is that they can induce the full attack on a recurrence without the usual help they would need from other cells. They are much faster in action than the regular travelling memory cells that have to get to the site. The traveling memory cells also produce more long term damage. Trm cells are able to tamp down and clear out the damaging inflammation when the attack is over.
There is some autoimmune damage from CD8+ cells related to the very specific virus antigen, but mostly not. If the other memory cells operate, there is much more diffuse damage.
T Regulatory (Treg) Cells in Brain Inflammation
 Ordinary T cells don’t produce autoimmunity in the brain. Only special cells like the FOXP3s. These can arise from ordinary peripheral T cells, however and protect against gut and lung infections, but not brain. Some Treg cells enter the brain and mature there and can fight inflammation.
Ordinary T cells don’t produce autoimmunity in the brain. Only special cells like the FOXP3s. These can arise from ordinary peripheral T cells, however and protect against gut and lung infections, but not brain. Some Treg cells enter the brain and mature there and can fight inflammation.
What appears to be happening is that peripheral T cells enter the brain with permission. There they mature, expand, and when activated again they can become regulatory inhibitory T cells as in other organs. It is a more elaborate procedure. They need some cytokine stimulation such as IL-10 and IL-33.
Basically, normally there is little Treg action in the brain. But when there is autoimmune response, they re-emerge in a different way to help.
T cells, Brain Function, and Dementia
 There is a lot more complexity in the interaction of brain and immune than triggering the “sick feeling” and stimulating new neurons in the hippocampus. This is just being uncovered.
There is a lot more complexity in the interaction of brain and immune than triggering the “sick feeling” and stimulating new neurons in the hippocampus. This is just being uncovered.
Microglia sense amyloid‐β stimulates tau tangles produce IL-1β and IL-23 for more inflammation. Now it is found that there are more types of CD4+ T cells related to amyloid 42, more common in Alzheimer’s. IFNγ‐producing T cells in choroid help decrease Alzheimer’s.
Many ordinary brain functions are related to T cell signaling, not just stress and depression. Memory and social behavior are related to signals from T cells IL-4, IIFNγ, and IL-17. IL-4 CD4+ T cells in meninges help learning by stopping inflammation type cells and by producing BDNF in astrocytes. This stimulates new neurons.
IFNγ from T cells in meninges helps circuits of social behavior by producing new neurons and increasing interactions of other brain cells. Choroid cells interact with new brain cells in hippocampus. In fetus, cytokines increase brain development.
Update of T Cell Complexity in the Brain
 T cells are the master regulators of the immune system, so it is not surprising they have many different roles in the brain. But, it has been found that they do a lot more than fight infections. They interact with brain cells for stress, cognition, “sick feeling, depression, Alzheimer’s and many other functions. For a long time, they were not considered to be in the brain—just a small number in the CSF.
T cells are the master regulators of the immune system, so it is not surprising they have many different roles in the brain. But, it has been found that they do a lot more than fight infections. They interact with brain cells for stress, cognition, “sick feeling, depression, Alzheimer’s and many other functions. For a long time, they were not considered to be in the brain—just a small number in the CSF.
Now research is showing many different possibilities about roles for a wide range of different types T cells, both in the brain and influencing the brain from elsewhere. This research is just beginning. The conversations among these many different kinds of T cells, along with other immune cells, and glia will determine treatments for many different brain diseases.