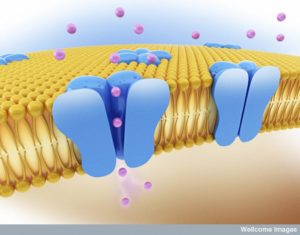
 Hundreds of large interacting protein molecules operate together at the synapse to send a signal from one neuron and trigger a reaction in the next neuron. Receptors are large complex protein molecules that sit in the membranes of neurons and respond to signals such as neurotransmitters. When triggered, the receptor produces a response that stimulates pathways and is often a cascade of events that eventually goes into the nucleus to alter genetic networks.
Hundreds of large interacting protein molecules operate together at the synapse to send a signal from one neuron and trigger a reaction in the next neuron. Receptors are large complex protein molecules that sit in the membranes of neurons and respond to signals such as neurotransmitters. When triggered, the receptor produces a response that stimulates pathways and is often a cascade of events that eventually goes into the nucleus to alter genetic networks.
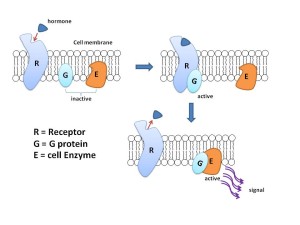
For many years, receptors on neurons have been considered to fall into two categories based on their structure and function. One forms a channel (ionic) where ions flow with a mechanism based on electrical charge. The other large class of receptors are molecules that trigger cascades of other molecules just below the membrane.
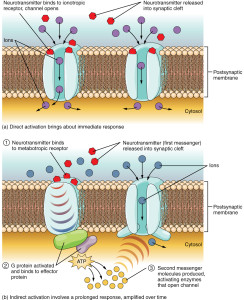 Ionic receptors (called inotropic) form channels with a structure of four proteins that cross through the membrane. By entirely crossing the membrane the receptor can interact with the stimulus outside of the cell and also allow ions to travel inside of the cell to alter electric balance. It can also send messages through the membrane with a part of the molecule that interacts inside of the cell.
Ionic receptors (called inotropic) form channels with a structure of four proteins that cross through the membrane. By entirely crossing the membrane the receptor can interact with the stimulus outside of the cell and also allow ions to travel inside of the cell to alter electric balance. It can also send messages through the membrane with a part of the molecule that interacts inside of the cell.
The other type (called metabotropic) has a protein that crosses back and forth through the entire membrane seven times forming a structure where a molecule binds on the outside of the cell. This triggers the pathways below through large complexes of proteins called G proteins. G proteins then trigger different pathways that can go to the nucleus to alter genes.
As everything in neurons (and cells in general), the situation just became more complex. New evidence shows that the ion channel types of receptors are much more complex and varied in their structures, interactions with molecules, and responses. In fact, it appears that parts of the ionic channel protein interact in a wide variety of ways with other cellular pathways. The distinction between these two categories of receptors is now much less clear.
Several of the most frequent neurotransmitters in the brain have both types of receptors—glutamate, GABA and acetylcholine.
Ion Channel Receptors Interact Widely
 Recently, with the ability to observe smaller and smaller molecular interactions, a large number of proteins have been found to interact with ion channel receptors. These are now seen to trigger a wide range of different responses that were previously only thought to occur with the other type (metabotropic).
Recently, with the ability to observe smaller and smaller molecular interactions, a large number of proteins have been found to interact with ion channel receptors. These are now seen to trigger a wide range of different responses that were previously only thought to occur with the other type (metabotropic).
One well known pathway triggered by ionic receptors is called Wnt, which triggers many other different pathways. The name “Wnt” once stood for something but is now just known by these initials since science has moved on from the original discovery. Wnt has pathways that go to the nucleus triggering genetic networks. Another regulates the skeleton scaffolding of the cell that determines shapes. And the third triggers levels of the ion calcium that has many different effects.
All of these work through varied different enzymes. Kinase enzymes place phosphorus energy molecules in a wide range of processes. In this Wnt pathway there are many different kinases affected that have wide ranging effects such as actin scaffolding and adhesion to other cells. All of these different functions occur independently and uniquely in the fetus. They become part of a unified pathway in adults. These different pathways are called canonical and non-canonical really based only on when they were discovered, further confusing this whole subject.
There are small differences between ion channel receptor molecular structures (such as a difference between GABA and acetylcholine receptors). But they are more similar than different with regions that are available for interaction on both sides of the membrane (inside and outside the cell). They form a channel that ions travel through. They have similar but slightly different characteristics such as what opens and closes the channels.
Now, with these new developments, there isn’t even a classification system for the differences in their cellular functions. Attempts to make such distinctions include listing those that open and close the channel (called canonical). They also must include those that alter the structures of the channel proteins. These triggers include molecular pathways that are similar to the metabotropic types (Such a new use of ion channels are called non canonical).
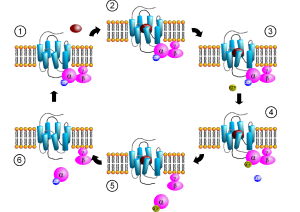 What is strange about the newly discovered latter option is that there is no obvious structure for the cascade, such as the G proteins in the other types of non-ionic receptors. To make this even more complex, there appear to be interactions of the channel molecule with other molecules than the neurotransmitter. This is called a “structural” or “scaffolding” mode of interaction and appears to be very significant for the function of brain circuits.
What is strange about the newly discovered latter option is that there is no obvious structure for the cascade, such as the G proteins in the other types of non-ionic receptors. To make this even more complex, there appear to be interactions of the channel molecule with other molecules than the neurotransmitter. This is called a “structural” or “scaffolding” mode of interaction and appears to be very significant for the function of brain circuits.
Glutamate Receptors: Kainate Type
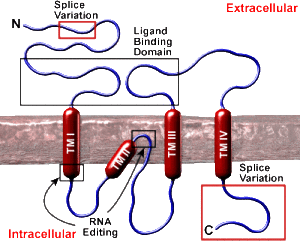 One of the group of ionic glutamate receptors is called kainite receptors (KARs). They are made of five distinct subunits that allow for a great number of variations. Sub units are attracted to glutamate in either a strong manner, which determines where the receptors are in the membrane and weak determining membrane response. Receptor variations are also determined by alternative messenger RNA splicing. Each type occurs on specific types of neurons, such as interneurons versus excitatory, and also being on pre and post synapse membranes. A confusing finding is that these can interact with pathways from metabotropic receptors.
One of the group of ionic glutamate receptors is called kainite receptors (KARs). They are made of five distinct subunits that allow for a great number of variations. Sub units are attracted to glutamate in either a strong manner, which determines where the receptors are in the membrane and weak determining membrane response. Receptor variations are also determined by alternative messenger RNA splicing. Each type occurs on specific types of neurons, such as interneurons versus excitatory, and also being on pre and post synapse membranes. A confusing finding is that these can interact with pathways from metabotropic receptors.
KARs are very vital to trigger the maintenance and release of neurotransmitters. They are part of the triggers for endocannabinoids (see post). They control glutamate release with both stimulation and inhibition. Some of these seem to involve G proteins to make matters even more confusing. There are now many different versions of this discovered.
One of the central ideas about neuron signaling involves cell excitability. When cells become excitable and are triggered, the action potential occurs. Another type of excitability occurs near the synapse where calcium channel trigger the vesicles with neurotransmitters. This involves the combined actions of a large number of channels. Excitability can be altered independently of the synapse and is an important unique mechanism of neuroplasticity. These involve many different potassium and calcium channels.
 A variety of neurotransmitters and other molecular factors alter and regulate the excitability through channel effects (ACH, norepinephrine, serotonin, etc). in many different parts of the brain circuits. The many sub unit variations determine which KARs are used in each of these situations. In the hippocampus many different KARs work to determine neuroplasticity for memory.
A variety of neurotransmitters and other molecular factors alter and regulate the excitability through channel effects (ACH, norepinephrine, serotonin, etc). in many different parts of the brain circuits. The many sub unit variations determine which KARs are used in each of these situations. In the hippocampus many different KARs work to determine neuroplasticity for memory.
KARs stimulate and inhibit the production of the axon initial segments. These seem to be based on whether the ions in the channels are in a high or low concentration. The properties are different in the fetus and the adult neurons. There are many examples where these ion fluxes trigger other non-canonical specific molecular signaling pathways.. Which is triggered is based on high or low concentrations of the ions.
 The number of dendrites have been found to be triggered by these same non-canonical mechanisms.
The number of dendrites have been found to be triggered by these same non-canonical mechanisms.
The problem with this is that it is very difficult to imagine how KARs channel differences could trigger these changes since they don’t have the clear mechanisms of G protein. Research is now looking for an intermediary molecule.
Glutamate Receptors: AMPA Types
Another major type of glutamate receptor is AMPA (or a-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid). This is the major pathway of fast excitatory brain circuits, which is most of the brain. There are four subunits that make channels and a large number of variations in the ways these ion channels work. Like KARs, there are many recently found to work by unusual mechanisms with mysterious factors.
 One of the effects involves factors that interact with and inhibit G proteins. They activate a vital large pathway called MAPK (or mitogen activated protein kinase), which is vital for signals to go into the nucleus and trigger genetic activity. Non-canonical effects (that is, ionic channels acting like metabotropic) affect gene activity, the excitability of the neuron, cell survival and the regulation of myelin production by oligodendrocytes.
One of the effects involves factors that interact with and inhibit G proteins. They activate a vital large pathway called MAPK (or mitogen activated protein kinase), which is vital for signals to go into the nucleus and trigger genetic activity. Non-canonical effects (that is, ionic channels acting like metabotropic) affect gene activity, the excitability of the neuron, cell survival and the regulation of myelin production by oligodendrocytes.
The most well-known activity is in control of the synapse. This has been seen in the cerebellum, the retina and elsewhere. They trigger BDNF, the vital factor producing new neurons in the hippocampus. As with KARs, AMPA can regulate by stimulation and inhibition of many of these non-canonical functions, triggered by high and low concentrations of ions affecting particular intermediary signaling molecules.
NMDA Receptors
 N-methyl-D-aspartate receptors are very prominent in memory circuits of the hippocampus. At rest, a magnesium molecule blocks the channel (pore) in the membrane. If the electrical status of the neuron’s membrane is altered (depolarized) by a trigger, then the magnesium moves out of the way and the channel is open. Calcium rushes in producing many different kinds of effects throughout the brain. It is especially important in learning and neuroplasticity. One region of the NMDA receptor molecule (NMDARs) is a scaffold that interacts with many other molecules and factors.
N-methyl-D-aspartate receptors are very prominent in memory circuits of the hippocampus. At rest, a magnesium molecule blocks the channel (pore) in the membrane. If the electrical status of the neuron’s membrane is altered (depolarized) by a trigger, then the magnesium moves out of the way and the channel is open. Calcium rushes in producing many different kinds of effects throughout the brain. It is especially important in learning and neuroplasticity. One region of the NMDA receptor molecule (NMDARs) is a scaffold that interacts with many other molecules and factors.
An unusual process occurs with recurrent stimulation of NMDARs. This process is triggered by an enzyme taking off a phosphate energy molecule from the receptor. The receptor is then taken out from the membrane (internalized), surrounded by a small membrane and eliminated or “eaten”.
Traditional views of neuroplasticity (see the post Fantastic Array of Neuroplasticity Mechanisms) showed that calcium increases from NDMAR stimulation are involved in strengthening (long term potentiation) or weakening (long term depression) of the synapse. This has been correlated with learning. But, now non-canonical signaling appears to be involved as well through the MAPK pathway already mentioned. Another finding is that in Alzheimer’s amyloid clumps also stimulate long term depression through this non canonical method.
Glutamate Receptors: Delta Types
 Glutamate delta receptors were discovered more recently than the others. These also have unusual mechanisms that involve stimulation and inhibition without a clear signal triggering the channel. In the cerebellum, neuroplasticity that produces motor learning (riding a bike, playing musical instrument, sports) needs stimulation of two systems at the same time, one of them the well-known climbing fibers. Many different proteins are found to interact with the receptors unrelated to the channel ions.
Glutamate delta receptors were discovered more recently than the others. These also have unusual mechanisms that involve stimulation and inhibition without a clear signal triggering the channel. In the cerebellum, neuroplasticity that produces motor learning (riding a bike, playing musical instrument, sports) needs stimulation of two systems at the same time, one of them the well-known climbing fibers. Many different proteins are found to interact with the receptors unrelated to the channel ions.
In the fetus, the receptor is altered when triggered by D-serine and this causes AMPAR receptors to be taken from the membrane, decreasing synapse function. In the adult, long term potentiation neuroplasticity occurs with proteins related to the scaffolding of microtubules alter the receptors for neuroplasticity.
Structural or Scaffolding Non Canonical Signaling
 The ionic channel receptors can have elaborate interactions by producing complexes consisting of many molecules in the scaffolding (microtubules and actin tubules). They are not really the same kind of signaling because there is no molecule that triggers the receptor. It is another entirely different side activity of the receptor. These are mainly triggered by glutamate Delta and AMPAR receptors.
The ionic channel receptors can have elaborate interactions by producing complexes consisting of many molecules in the scaffolding (microtubules and actin tubules). They are not really the same kind of signaling because there is no molecule that triggers the receptor. It is another entirely different side activity of the receptor. These are mainly triggered by glutamate Delta and AMPAR receptors.
One important example occurs in the cerebellum. There are two kinds of excitatory axon attachments to the synapse. One type attaches to dendrites near the cell body and another at a distance. In this process, many are pruned until there are the same number of each. They then compete as part of a very complex process that produces more vesicles for the synapse.
Another version of this structural interaction with the channel receptors occurs with AMPAR in the hippocampus. There, a scaffold is built that increases dendrite spines and generally the function of the synapse.
Nicotine Acetylcholine Receptors
 These are channels for receptors that produce rapid signaling for cognition. Neurons, blood cells, and immune cells (leukocytes) have them. The non-canonical functions were first discovered in the leukocytes. In T cells these channel receptors can increase calcium signaling without alterations in the calcium flow of ions in the channel. Instead it produces a protein complex including other receptors and pathways with kinase enzymes that release calcium.
These are channels for receptors that produce rapid signaling for cognition. Neurons, blood cells, and immune cells (leukocytes) have them. The non-canonical functions were first discovered in the leukocytes. In T cells these channel receptors can increase calcium signaling without alterations in the calcium flow of ions in the channel. Instead it produces a protein complex including other receptors and pathways with kinase enzymes that release calcium.
In monocytes another mechanism of this type involves the vital cytokine interleukin-1b (IL-1b). There are, in fact, two mechanisms that release the cytokine, one by a receptor that alters the ion flow and the other without.
This same signaling occurs in neurons in circuits of hippocampus and striatum. This affects glutamine and GABA circuits via the acetylcholine receptors.
Non Canonical Effects Appear to Be Subtle Ad On to Canonical
 Almost all of the ion channel receptors (except GABA so far) have both of these types of signaling. They all have the same basic structure with many variations. Several of the subunits can connect to G proteins that trigger cascades. All of these examples found thus far appear to be subtle effects, rather than the predominant effects. There appear to be great similarities in the mechanisms thus discovered for different receptors, such as KARs and NMDARs and then Deltas and NMDARs.
Almost all of the ion channel receptors (except GABA so far) have both of these types of signaling. They all have the same basic structure with many variations. Several of the subunits can connect to G proteins that trigger cascades. All of these examples found thus far appear to be subtle effects, rather than the predominant effects. There appear to be great similarities in the mechanisms thus discovered for different receptors, such as KARs and NMDARs and then Deltas and NMDARs.
Does this mean that they are related through evolution? Each receptor evolved for different specific purposes. AMPARs are prominent in rapid axon potentials. NMDARs have particular effects on neuroplasticity and strengthening or weakening specific synapses in circuits. KARs seem to be best at modulating the release of transmitters at the synapse.
Brain Receptors Just Got Even More Complex
 Up until now, ion channels have been vital in synapses, neuroplasticity, learning, memory and cause of diseases. All mechanisms have related to the characteristics of ion channels. Now, this has to be rethought since almost all of the distinct mechanisms also include a subtle but relevant non canonical mechanism that is completely different.
Up until now, ion channels have been vital in synapses, neuroplasticity, learning, memory and cause of diseases. All mechanisms have related to the characteristics of ion channels. Now, this has to be rethought since almost all of the distinct mechanisms also include a subtle but relevant non canonical mechanism that is completely different.
These non-canonical processes involve molecules and factors interacting in different and unique ways with the complex molecule that sits in the membrane and has a surface both outside and inside the cell. In some ways they are like G proteins, but without a clearly understood mechanism. This is yet another factor that greatly raises the complexity of receptors and brain function.