 This is the second post on brain cannabinoids. The previous post described the vast functions of endogenous cannabinoids in the developing brain. That post, also, described how cannabinoids are critical for stimulating neuronal stem cells in the adult brain’s hippocampus related to learning and memory. To fully understand what is currently known about cannabinoids in the brain, both this post and the previous post should be read.
This is the second post on brain cannabinoids. The previous post described the vast functions of endogenous cannabinoids in the developing brain. That post, also, described how cannabinoids are critical for stimulating neuronal stem cells in the adult brain’s hippocampus related to learning and memory. To fully understand what is currently known about cannabinoids in the brain, both this post and the previous post should be read.
This will discuss many complex cannabinoid functions in the adult brain. It will, also, focus on what is known about cannabinoids in inflammation and the aging brain.
Cannabinoid signaling is highly related to both neuro-inflammation and neurodegenerative disorders. (See post on Inflammation Pathways in Neuroplasticity). In fact, a new cannabinoid treatment for multiple sclerosis was just approved. This medicine, nabiximol, is a combination of THC and cannabidiol. THC has been used previously for vomiting and weight loss in cancer and AIDS patients. But, this new medication is the first cannabinoid to treat the fundamental cause of a major degenerative disease. With increasing research, it is now clear that the functions and signaling of cannabinoids change with both age and specific brain diseases. They are part of a large number of poorly understood derivatives of fatty acids, many of which have biological actions related to brain function, aging, inflammation and degenerative brain disease.
Brief Summary of Cannabinoid 
For many more details on cannabinoids in the developing brain, please read the previous post.
Endocannabinoids are derived from the omega 6 fatty acid arachidonic acid, which is bound to cellular membranes and cut off in a very complex process. “Endo” refers to cannabinoids made in the brain, as opposed to “phyto-cannabinoids” made in plants and ingested. For simplicity, these posts on the brain use the general term “cannabinoids.”
The most important and abundant brain cannabinoid is 2-AG—2 2-arachidonoylglycerol—and the second is AEA—anandamide or N-arachidonoylethanolamine. They stimulate the two cannabinoid receptors CB1 and CB2. While there are 70 different cannabinoids found in plants, THC is the only one so far that stimulates CB receptors.
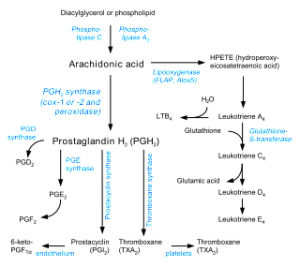
Major neurotransmitters trigger cutting off the fatty acids from the membrane to start manufacturing cannabinoids. Receptors that stimulate cannabinoid manufacture include dopamine, acetylcholine, serotonin and glutamate NMDA. One well-known mediator in this process is the important immune molecule prostaglandin made by the enzyme COX2 (cyclooxygenase).
There are a wide range of cannabinoid effects in the brain. They are important for pain sensation, appetite, memory, motor learning, neuroplasticity and mood. The CB receptors, also, trigger the peripheral nervous system and the immune system.
- Cannabinoid are critical for controlling metabolic systems.
- Cannabinoids are involved in the complex regulation of insulin, the cortisol stress response and the hypothalamus-pituitary-adrenal system.
- In the immune system, cannabinoids modulate both neurons and immune cells.
 In the fetus. cannabinoid signals determine the creation of new neurons and glia from stem cells; the neuronal polarity for migration and axon production; and the formation of the synapses in the cortex. They, also, build the pattern of the structure of neuronal connections. The cannabinoid signals are critical to manufacture new neurons for the developing brain and the structures that they form. Cannabinoids, also, signal widely to stimulate many glia cells. They build the basic sensory long tracts between the cortex and the thalamus. They are critical for embryo implantation and continued survival of the fetus.
In the fetus. cannabinoid signals determine the creation of new neurons and glia from stem cells; the neuronal polarity for migration and axon production; and the formation of the synapses in the cortex. They, also, build the pattern of the structure of neuronal connections. The cannabinoid signals are critical to manufacture new neurons for the developing brain and the structures that they form. Cannabinoids, also, signal widely to stimulate many glia cells. They build the basic sensory long tracts between the cortex and the thalamus. They are critical for embryo implantation and continued survival of the fetus.
In adults, cannabinoids have different functions than in the child. Therefore, effects of ingested plant cannabinoids are very different in the child and the established adult brain.
Cannabinoids in the adult maintain neuroplasticity and keep stem cells ready to make new neurons for memory. They stimulate rapid response to brain injury by triggering some of the processes that originally built the brain. They are highly related in complex ways to degenerative brain disease, inflammatory brain disease and depression. Cannabinoids provide stimulus for new neurons and directions for their travel in the brain including a mechanism in the cure of depression.
The very malignant cancer glioblastoma is related to lipids in the membrane. In animals, cannabinoids have stopped these cancers of glia cells through stimulation of cell death of tumor cells without hurting normal cells.
Making Cannabinoids
 What is most surprising in the recent research is the adaptability of the cannabinoid system. The various molecules are called up in local areas and in specific circumstances. For example, the previous post described a unique way that both the cannabinoids and its receptors mysteriously appear in stem cells at the same time and work to stimulate the stem cell’s activity in making new neurons.
What is most surprising in the recent research is the adaptability of the cannabinoid system. The various molecules are called up in local areas and in specific circumstances. For example, the previous post described a unique way that both the cannabinoids and its receptors mysteriously appear in stem cells at the same time and work to stimulate the stem cell’s activity in making new neurons.
The metabolism of cannabinoids is very complex and only now being discovered. They are part of pathways that include many other biologically active fatty acid derivatives. Important enzymes in these pathways are triggered by calcium to manufacture the two cannabinoids from two different fatty acid derivatives—NAPE (N-acyl-phosphatidyl-ethanolamine) for AEA and DAG (diaglycerols) for 2-AG.
Both 2-AG and anandamide are hydrolyzed by the well-known enzyme COX2 (cyclooxygenase or prostaglandin synthase). Other enzymes that degrade cannabinoids are DAG lipases, called DAGLs. These are involved in the mechanism where cannabinoids use retrograde inhibition from postsynaptic neurons, after 2-AG stimulates CB1 on the pre synaptic neurons (see previous post). There are other less well-known enzymes that metabolize cannabinoids and related fatty acid derivatives.
 In the adult, CB receptors are widely placed in the brain. CB1 is most common in inhibitory (GABA) and excitatory (glutamate) synapses of major brain regions such as the hippocampus, cortex, basal ganglia and cerebellum. CB2 is not common in the normal brain, but develops in glia cells during neuro-inflammation.
In the adult, CB receptors are widely placed in the brain. CB1 is most common in inhibitory (GABA) and excitatory (glutamate) synapses of major brain regions such as the hippocampus, cortex, basal ganglia and cerebellum. CB2 is not common in the normal brain, but develops in glia cells during neuro-inflammation.
The actions of the receptors are different when they are in different types of neurons and in different compartments of the cells. For example, CB1receptors behave differently when they are in glutamate and GABA neurons. Cannabinoids, also, stimulate many other receptors, including TRPV1 and PPAR?.
Changes in Cannabinoid Signals in Youth and Aging
 In animal studies, CB1 receptors are greatest in the young and stay at that level until they decrease in aging. The peaks are at puberty with 50% less in the elderly. This decline occurs in the cerebellum, cortex, hippocampus and hypothalamus. The change is related to production of the receptors by genetic networks and affects the triggering cascades.
In animal studies, CB1 receptors are greatest in the young and stay at that level until they decrease in aging. The peaks are at puberty with 50% less in the elderly. This decline occurs in the cerebellum, cortex, hippocampus and hypothalamus. The change is related to production of the receptors by genetic networks and affects the triggering cascades.
Also, the levels of enzymes that metabolize cannabinoids change—some increase and some decrease in humans and rodents. In baby rodents, CB1 is not necessary. But, by adolescence, they are necessary for cognition. Adult rodents with less cannabinoids do very poorly.
With aging animals, sometimes there are no cannabinoids. Without CB1, GABA neurons are altered but not glutamate. These changes occur in the brain and skin and not elsewhere. Both receptors are necessary for myelin production and CB1 for migration of neurons and axons as well as synapses (see previous post).
With less CB1 function in aging, there is neuron loss and more neuro-inflammation. With less CB1 there are decreased hippocampus neurons and signs of inflammation including more astrocytes, microglia and inflammation cytokines. Less CB2, on the other hand, affects the bodily organs and not the brain. It causes bone loss and other organs.
If cannabinoids run out, the creation of new neurons from stem cells stops. If an experiment blocks the breakdown of cannabinoids and increases the creation of new cannabinoids, new neurons are made.
Inflammation in the Brain and Cannabinoids
 Both aging and some brain diseases alter cannabinoid pathways and their effects. The pathways connecting cannabinoids to aging are extremely complex and are just now being discovered. They involve many different metabolites of cannabinoids and many other receptors along with CBs.
Both aging and some brain diseases alter cannabinoid pathways and their effects. The pathways connecting cannabinoids to aging are extremely complex and are just now being discovered. They involve many different metabolites of cannabinoids and many other receptors along with CBs.
Immune cells that invade the brain produce receptors for cannabinoids. Microglia normally have a small amount of CB2 receptors. In animal models of multiple sclerosis, microglia have 200 times more receptors. But, it is not this large amount of microglia receptors that causes much of the problem. Rather, it is a small number of T cells entering the brain with CB2 receptors that stimulates the pathology. T cells stimulate the neuro-inflammation damage that is seen in multiple sclerosis like illness in animals.
In humans with multiple sclerosis, there are changes in levels of cannabinoids. But, this is, also, true in Parkinson’s, Huntington’s and ALS, as well as animal models of these diseases. What is not known is exactly how each cell—neuron, microglia, astrocyte, and T cell—responds to the particular changes of cannabinoids in different stages of aging.
With stress and inflammation, cannabinoids are stimulated, which are part of the balancing act of two opposite forces involving cannabinoids. Cannabinoids are critical for counteracting and producing the inflammation pathways. Cannabinoids are critical in aging and in brain inflammation, but have very complex and sometimes opposite effects.
Cannabinoids and Aging
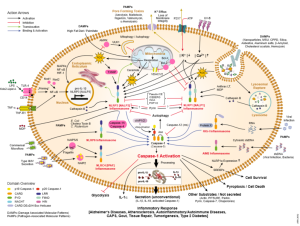
Some research has found that cells alter their cannabinoid signaling with aging. One example is in immune cells that produce increased inflammation cytokine signals, such as the powerful NF-??. These signals produce specific inflammatory proteins triggered by various cannabinoid pathways and signals. Some hypothalamus pathways contribute to inflammation and some fight against it. Cannabinoids trigger the stress response of the hypothalamus with more cortisol fighting against inflammation. How these opposite pathways interact can have important results for aging, but they are not yet clear.
In brain alterations of aging and brain inflammation, cannabinoid responses are disrupted. Taking THC for a long time increases the number of microglia, which can have opposite affects on inflammation. The brain changes that alter the hypothalamus inflammation response, also, affect muscles, bone, tendons and skin, as well as cognitive ability. The hypothalamus inhibits the NF-?? cytokine that increases inflammation. In mice, if this is counteracted, they have increased cognition and they live longer.
Cannabinoids have two critical functions in stress responses. One is that they mediate the hypothalamic responses and the other involves the use of energy in the body organs. CB1 receptor stimulation stops use of energy through orexin. The cannabinoids in the hypothalamus are stopped by leptin, which decreases appetite and increases inflammation. Both orexin and leptin are critical in appetite and body weight.
Cannabinoids are disrupted by high fat diets that increase eating and decreased metabolism. These changes cause more glia cells, increasing aging affects and neuro inflammation. Normally, cannabinoids would counteract inflammation with decreased efficacy as the animal ages.
Cannabinoids and Metabolism
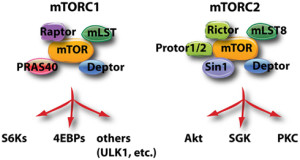 Cannabinoids influence a wide variety of molecules that are part of the basic metabolic pathways. Other posts have described how metabolic pathways in microbes and human cells serve a dual purpose of basic energy and molecular synthesis, and, also, as critical signals in pathways that cascade to the nucleus triggering genetic networks (see posts How do Microbes Make Decisions and T cells Critical in Cognition). These pathways are very important in cellular and organism aging.
Cannabinoids influence a wide variety of molecules that are part of the basic metabolic pathways. Other posts have described how metabolic pathways in microbes and human cells serve a dual purpose of basic energy and molecular synthesis, and, also, as critical signals in pathways that cascade to the nucleus triggering genetic networks (see posts How do Microbes Make Decisions and T cells Critical in Cognition). These pathways are very important in cellular and organism aging.
Cannabinoids interact with the very complex actions of mTOR. A previous post described the pathways connected with mTOR, which senses nutrients and regulates energy metabolism. (see post The Very Intelligent Protein mTOR). mTOR controls protein synthesis in a variety of ways, especially by triggering and stopping ribosome translation of messenger RNA into proteins. mTOR is, also, noted to control neuroplasticity, axons, dendrites and synapses.
THC stimulates CB1, which triggers mTOR activity in many major brain centers, including hippocampus, striatum, cerebellum, amygdala and frontal cortex. The cannabinoid effect on mTOR is highly related to cognition and THC’s affects on cognition are mediated by mTOR. There are examples of diseases, like Fragile X, where increased cannabinoids are part of disrupted pathways stimulating mental impairment. In aging, these effects are more prominent.
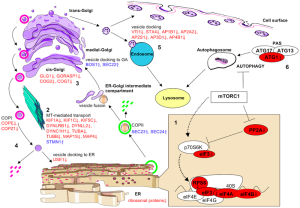
mTOR’s actions, also, influence autophagy, the programed dismantling of damaged cellular compartments and material. In autophagy these damaged substances are placed in vesicles and sent to lysosomes for destruction. The alterations in autophagy with aging affect the removal to mis-folded proteins in lysosomes and contribute to degenerative brain diseases.
Autophagy, also, provides nutrients from the cycled materials, which triggers mTOR. Studies in animals show that inhibiting mTOR increases autophagy, which increases longevity. Cannabinoids, also, trigger autophagy in cancer cells increasing their life through a different mechanism than mTOR. Without cannabinoids, cells accumulate more damaging chemicals from faulty autophagy.
Cannabinoids and Brain Diseases
 Cannabinoid mechanisms of aging and autophagy are related to Huntington’s disease. Early in the disease there is less stimulation of CB1 receptors. This causes damage to the striatal brain regions, the one most affected by Huntington’s. Loss of these receptors in aging and cannabinoid effects on mTOR can increase this problem.
Cannabinoid mechanisms of aging and autophagy are related to Huntington’s disease. Early in the disease there is less stimulation of CB1 receptors. This causes damage to the striatal brain regions, the one most affected by Huntington’s. Loss of these receptors in aging and cannabinoid effects on mTOR can increase this problem.
In Alzheimer’s disease, lysosome alterations can increase amyloid-? in the cortex. Cannabinoid pathways in this circumstance are very complex and not well understood. They involve CB receptors at the membrane and inside the cell, regulating the relation of internal organelles including lysosomes and mitochondria. This type of internal cellular signaling is very unusual.
Many CB1 receptors are on lysosome and mitochondria membranes. The mitochondria receptors are involved in regulation of the energy production by affecting the mitochondria complex I. These receptors provide feedback inhibition of the behavior at the synapse related to energy. Alterations of mitochondria due to aging, also affect these processes.
Changes in cannabinoids, also, influence ALS and Parkinson’s.
Very Complex Relation of Cannabinoids and Brain Degeneration
 In the pathways that metabolize cannabinoids there are many similar molecules that have biological effects without being able to trigger CB receptors. They use the same enzymes as cannabinoids for manufacture and metabolism. These other molecules (only recently being described) stimulate other receptors, which are common on many different cells. They, also, influence inflammation and brain disease. Therefore, the situation in degenerative disease is very complex.
In the pathways that metabolize cannabinoids there are many similar molecules that have biological effects without being able to trigger CB receptors. They use the same enzymes as cannabinoids for manufacture and metabolism. These other molecules (only recently being described) stimulate other receptors, which are common on many different cells. They, also, influence inflammation and brain disease. Therefore, the situation in degenerative disease is very complex.
Molecules derived from fatty acids and related to cannabinoids can include an amino acid (lipoaminoacids). These are, also, called amine transmitters—versions of dopamine and serotonin are called N-acyldopamines, N-acylserotonins and N-arachidonoyldopamine, N-arachidonoylserotonin. These are only now being researched but are related to neuro-inflammation and toxicity from too much excitatory glutamate. They influence special calcium channels called Ca3.2 and Ca3.3—channels involved in neuroplasticity, which decrease in aging. The special molecules mentioned above block these channels affecting plasticity and memory.
To make this even more complex, the two cannabinoids are oxidized by multiple fatty acid enzymes (such as the famous COX2) and create more molecules that stimulate even more unusual receptors that affect inflammation. Derivatives of prostaglandin have receptors that trigger signaling cascades different from cannabinoid and prostaglandin receptors.
 These complex recently discovered cascades are related to changes in Huntington’s, Alzheimer’s and Parkinson’s. A metabolite of cannabinoid produced by the enzyme COX2 is involved in increasing inflammation in sensory neurons. In Alzheimer’s, blocking enzymes related to prostaglandin causes more inflammation and affects amyloid plaques. These effects do not utilize CB receptors. Very complex opposing actions of many of these little understood fatty acid intermediaries can either increase or decrease inflammation and increase or decrease cognition.
These complex recently discovered cascades are related to changes in Huntington’s, Alzheimer’s and Parkinson’s. A metabolite of cannabinoid produced by the enzyme COX2 is involved in increasing inflammation in sensory neurons. In Alzheimer’s, blocking enzymes related to prostaglandin causes more inflammation and affects amyloid plaques. These effects do not utilize CB receptors. Very complex opposing actions of many of these little understood fatty acid intermediaries can either increase or decrease inflammation and increase or decrease cognition.
To totally confuse the entire picture, recent research shows that before cannabinoids are metabolized into these other molecules, they also, affect other receptors that are not CBs. TRPV1 is best known receptor of this type and is significant in neurons and glia cells, both in toxicity from excitatory neurons and brain inflammation. These affects can help or hurt the symptoms of Parkinson’s and Huntington’s. Another cannabinoid effect is found to increase GABA receptors, affecting aging and brain degeneration by positively and negatively affecting neuroplasticity. All of these diverse pathways are highly related to exact timing and exact locations in the cells.
There appears to be a vast complex web of cascades related to metabolites of cannabinoids and other fatty acids that are just now being discovered. Some have called it the “cannabinoidome”, which includes many fat signals, enzymes and receptors. These signals operate in very rapid time frames and in very specific places in the cell. They have very different properties than giving synthetic cannabinoids like THC.
Some of the research molecules, for example, give opposing effects, including the positive action of stopping spasticity in multiple sclerosis. Other molecules have been less successful in Parkinson’s, Huntington’s, ALS and Alzheimer’s. One example is CB1 and 2 having opposite effects in ALS. Another is CB1 on inhibitory and excitatory neurons. There is reason to believe that when they are better understood some substances might help these diseases
Cannabinoids in Inflammation and the Aging Brain
 While cannabinoids are critical in the adult brain, as well as the developing brain, there is much to be learned. In the adult, cannabinoids stimulate new neurons for learning and memory. Cannabinoids are, also, altered in aging and are deeply involved in brain inflammation and degenerative brain diseases.
While cannabinoids are critical in the adult brain, as well as the developing brain, there is much to be learned. In the adult, cannabinoids stimulate new neurons for learning and memory. Cannabinoids are, also, altered in aging and are deeply involved in brain inflammation and degenerative brain diseases.
Complicating current research is that after the free fatty acids are cut from the cell’s membrane, they are metabolized into a large number of different molecules including the two major brain cannabinoids. Many of these other less understood metabolites are, also, active in brain processes, inflammation and brain disease. While currently very confusing, research into the interaction of cannabinoids and a wide range of other lipid signalling molecules in adult brain function, aging, neuro-inflammation and brain disease is a very exciting field with a lot of promise.
As with many other posts, describing monumental complexity in fatty acid signaling raises the question as to where the direction can be for all of this? For example, inside stem cells cannabinoids synthesis is suddenly triggered and at the same time a receptor appears in the same cell. The cannabinoid triggers the receptor, which stimulates the stem cell to make neurons. This happens as a response to a mental event of learning and memory.
How did the stem cell know that it was time to make more neurons in that part of the brain? The mental event is somehow related to this molecular action, which raises the question of mind interacting with molecules.
