 The neuron’s stable but ever changing structure is quite remarkable. While maintaining its shape, it performs specialized functions, such as growing long axons to send signals to other cells and growing thousands of dendrites to receive information from other cells. How does the engineering work?
The neuron’s stable but ever changing structure is quite remarkable. While maintaining its shape, it performs specialized functions, such as growing long axons to send signals to other cells and growing thousands of dendrites to receive information from other cells. How does the engineering work?
Regulation of the changing internal structure
Each human cell has over a billion individual protein molecules and 10% are involved in signaling information. Finding the appropriate mate for a signaling molecule is a monumental problem. It is even more difficult in the extremely long neuron.
Signaling is possible with such a complex array of molecules because processes occur in specific locations. One major mechanism of the cell to accomplish this is the compartments or organelles in the cell. These very specialized regions have very specific machinery.
Another method involves specific locations on membranes. Signaling from outside of the cell occurs through receptor molecules in the membrane that are attached to scaffolding molecules on both intercellular and intracellular side.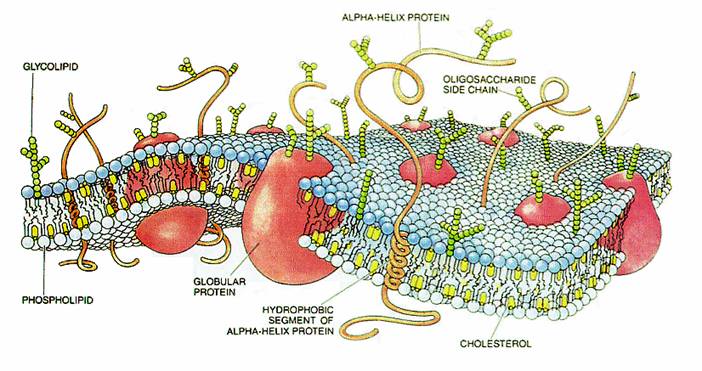
The third major way is through structures throughout the cell built by scaffolding molecules (see picture below.) These scaffolding structures are involved in housing and controlling the cell’s important processes and form a type of signaling language that determines outcomes for processes in the cell. Assembly line folding of proteins involves scaffolding to hold molecules in shape. Scaffolding proteins are integral to intracellular signaling including at synapses. They are also integral to the structures in the space between the cells at the synapse.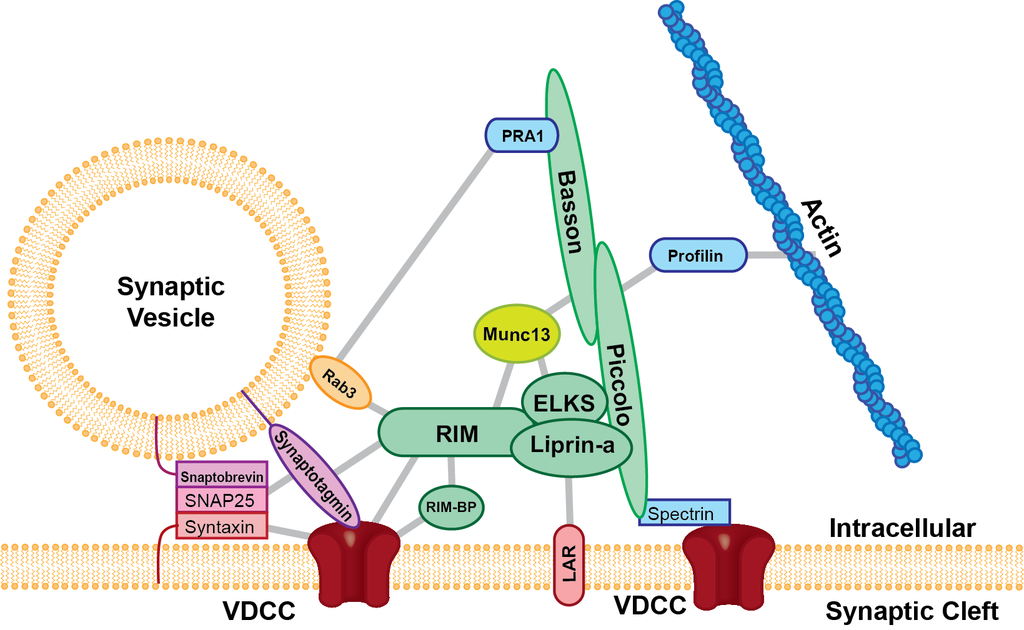
More and more evidence suggests that scaffolding proteins are critical in regulating where and when the components of the cell operate. Scaffolding proteins form three-dimensional Lego-like structures and protein machines that allow neural function to rapidly change.
Synapses are Built, Maintained, & Rebuilt with Scaffold Molecules
A previous post described that the building and pruning of synapses is a very active and rapid process that occurs moment by moment. Recent evidence shows synapses can even change nightly. To build a dendrite or axon, the outside membrane of the neuron must expand while building supports to control the new structure simultaneously. The dendrite can change daily, which may involve rapidly taking apart this entire structure. There are many more dendrites than axons, but at the tip of the axon, which is thousands times larger than the cell body, it separates into many terminal buds. All of these activities are directed, and implemented by scaffolding molecules constantly building, and changing support structures.
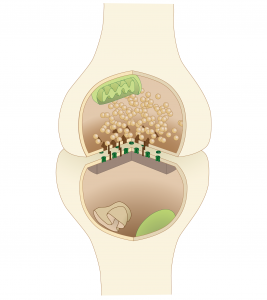 At the synapses, signaling molecules called neurotransmitters are released and reabsorbed by sacs that merge with and split from the cell membrane. A previous post described the fact that this merging and splitting of the sacs from the membrane does this without making holes in the membrane. This mechanism is maintained by hundreds of very active scaffolding proteins and enzymes. (see diagram below of three different types of vesicle release at the membrane supported by scaffolding molecules – this diagram is overly simplified and actually involves hundreds of scaffolding molecules)
At the synapses, signaling molecules called neurotransmitters are released and reabsorbed by sacs that merge with and split from the cell membrane. A previous post described the fact that this merging and splitting of the sacs from the membrane does this without making holes in the membrane. This mechanism is maintained by hundreds of very active scaffolding proteins and enzymes. (see diagram below of three different types of vesicle release at the membrane supported by scaffolding molecules – this diagram is overly simplified and actually involves hundreds of scaffolding molecules)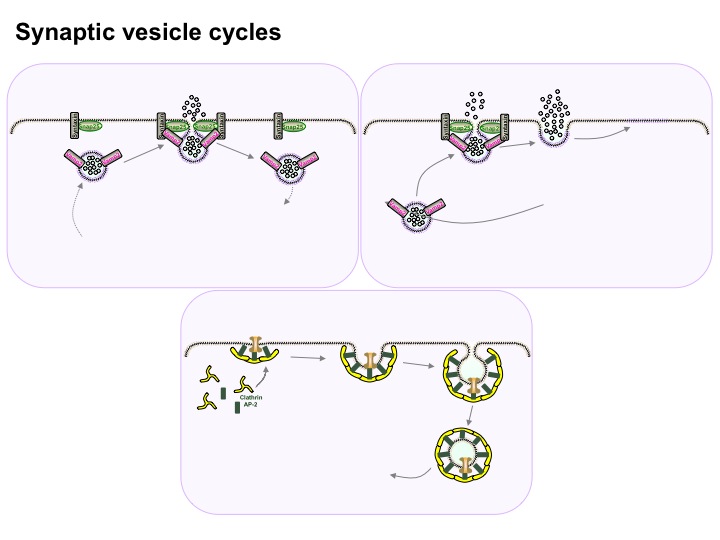 . In the dendrites, new receptor molecules are always being created and degraded. In order for this to happen, the cell has to rapidly transport enormous amounts of material to form the receptor molecules, sacs, and the neurotransmitters. All of this transport is done with motors along scaffolding molecules.
. In the dendrites, new receptor molecules are always being created and degraded. In order for this to happen, the cell has to rapidly transport enormous amounts of material to form the receptor molecules, sacs, and the neurotransmitters. All of this transport is done with motors along scaffolding molecules.
Also remember that in terms of scale, if the cell body is the size of a human being, the axon would be several inches thick but as long as a mile. Transportation of all working material has to be along this entire way on scaffolding tracks.
Neuron’s Three Lego Blocks
There are thousands of different types of scaffolding molecules in human cells. In neurons there are three important basic important types of building blocks, each with different capacities, that work together for the many rapid difficult scaffolding jobs. With these three molecules thousands of different complex lattice structures can be built. 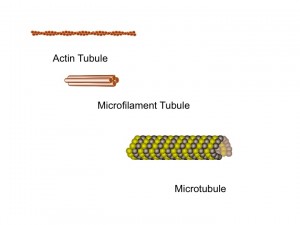
The first molecule, actin, which is also used in muscle, can build a membrane’s moving edge. Actin comes into play when a neuron grows a dendrite or axon.
The simplest, most flexible scaffolding molecules are called microfilaments or intermediate filaments which have branching capabilities that maintain their molecules’ flexibility and strength.
The strongest, most complex building block is the microtubule. It has an elaborate array of helper molecules that keep it always building or disassembling. The microtubule is built as a spiral cylinder with a positive charge on the leading edge of its growing spiral. Having a directional charge allows motor proteins to use it as a kind of railroad track to bring all types of molecules along the neuron. The axon of the nerve that runs from the spinal cord to the foot is two feet long, a monumental distance when you consider how small cells are.
 The microtubule holds a reliable shape and allows motors to shuttle molecules, and structures like sacs and mitochondria, along its entire route. This is important, as structures such as vesicle sacs, mitochondria, and messenger RNA, are synthesized near the
The microtubule holds a reliable shape and allows motors to shuttle molecules, and structures like sacs and mitochondria, along its entire route. This is important, as structures such as vesicle sacs, mitochondria, and messenger RNA, are synthesized near the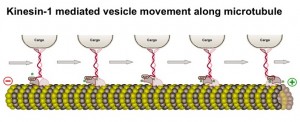 center of the cell but must be transported the entire two feet to the end of the axon. There are many motors that use these tracks but two major ones in the neuron are the dynein motor protein, which moves material toward the cell center, and the kinesin motor protein, which moves away from the cell center toward the synapse. (see diagram above)
center of the cell but must be transported the entire two feet to the end of the axon. There are many motors that use these tracks but two major ones in the neuron are the dynein motor protein, which moves material toward the cell center, and the kinesin motor protein, which moves away from the cell center toward the synapse. (see diagram above)
Three blocks and a computer
 Neurons’ ability to communicate, and therefore our ability to process information, depends entirely upon the ever-changing connections of axons and dendrites; these connections are organized and regulated by tubules. It appears that individual scaffolding molecules regulate some of the other tubules in a structure, but it is not at all clear how the three dimensional direction occurs which is vital for all scaffolding structures. Three dimensional direction is also critical to the spatial placement of dendrites which is also directed by the tubules. New research has shown that the spatial placement of dendrites on neurons is a critical aspect of memory and new learning with new links occurring very close by each other in specific patterns to emphasize the learning.
Neurons’ ability to communicate, and therefore our ability to process information, depends entirely upon the ever-changing connections of axons and dendrites; these connections are organized and regulated by tubules. It appears that individual scaffolding molecules regulate some of the other tubules in a structure, but it is not at all clear how the three dimensional direction occurs which is vital for all scaffolding structures. Three dimensional direction is also critical to the spatial placement of dendrites which is also directed by the tubules. New research has shown that the spatial placement of dendrites on neurons is a critical aspect of memory and new learning with new links occurring very close by each other in specific patterns to emphasize the learning.
Literally every function of the neuron depends upon the tubule structures. They have been called the “hubs for controlling the flow of cellular information.” Because of this, some scientists suspect there might be a structure directing the microtubules inside them at the quantum scale. In any case, something must direct these massively complex, shifting structures. Does a neuron think with its microtubules? How does this process connect with human thinking?
 The control of information performed by the tubular structures occurs in all cells, but is uniquely important in the neuron, where scaffolding molecules are constantly and rapidly being built and rebuilt. The neurons are the information conduits of the brain, in the form of budding spatially placed dendrites, changing connections between neurons and timed rapid signaling with neurotransmitters. All of these functions are performed using the scaffolding language, a LEGO system directed by a computer.
The control of information performed by the tubular structures occurs in all cells, but is uniquely important in the neuron, where scaffolding molecules are constantly and rapidly being built and rebuilt. The neurons are the information conduits of the brain, in the form of budding spatially placed dendrites, changing connections between neurons and timed rapid signaling with neurotransmitters. All of these functions are performed using the scaffolding language, a LEGO system directed by a computer.
The Circuit Board of the Neuron’s Brain
 A recent review article in Science described scaffold proteins as the “circuit boards” of the cell. This article noted that the scaffolding language is modular and therefore allows evolution to develop new pathways using the same genes and the same parts by simply rearranging the scaffolding structures.
A recent review article in Science described scaffold proteins as the “circuit boards” of the cell. This article noted that the scaffolding language is modular and therefore allows evolution to develop new pathways using the same genes and the same parts by simply rearranging the scaffolding structures.
The scaffolding proteins appear to be an engineering language of information flow that immediately responds to mental challenge with changing and evolving physical structures. Are these scaffolding tubules the “brain” inside the neuron? How can mental processes such as attention that alter the neuron’s function instantly affect this structural language of scaffolding molecules to direct rapid changes in dendrites, axons and synapses?