 Several intelligent viruses have been featured in previous posts. Herpes has a very complex life style with more than 70 genes—traveling up and down the neuron and in and out of the skin cell. HIV has an extraordinary set of complex behaviors with only 9 genes—travelling with critical proteins in its capsid, evading immune cells with multiple techniques and manipulating the cell’s complex nuclear machinery. How all of this can be accomplished with only 9 genes is not clear.
Several intelligent viruses have been featured in previous posts. Herpes has a very complex life style with more than 70 genes—traveling up and down the neuron and in and out of the skin cell. HIV has an extraordinary set of complex behaviors with only 9 genes—travelling with critical proteins in its capsid, evading immune cells with multiple techniques and manipulating the cell’s complex nuclear machinery. How all of this can be accomplished with only 9 genes is not clear.
Now the very intelligent Ebola virus takes front and center, with a complex, very deadly lifestyle and only 7 genes. Ebola virus builds a huge vessel for travel and uses many different attachment techniques for each human cell type. It tricks the cell into stimulating a very unusual entry and evades immune attacks in many different ways. Ebola virus builds a special decoy to avoid the immune system and, then, assembles new viruses with the help of complex raft structures floating in the cell’s membrane.
 Recent research has discovered that the Filo family of viruses (Filo because they look like threads or filaments) is very ancient—at least 20 million years old. Ebola has several related viruses—Marburg and Cuevavirus—and five subtypes—Zaire, Sudan, Bundibugyo, Tai Forest and Reston (monkeys in Virginia).
Recent research has discovered that the Filo family of viruses (Filo because they look like threads or filaments) is very ancient—at least 20 million years old. Ebola has several related viruses—Marburg and Cuevavirus—and five subtypes—Zaire, Sudan, Bundibugyo, Tai Forest and Reston (monkeys in Virginia).
The first discovery was Marburg in 1967. A decade later two of the Ebola strains were discovered. There have been 30 outbreaks of Ebola virus strains since then. Ebola has been found in bats, monkeys and pigs but the exact route from animals to humans is not certain. Everyone is now aware of the largest and most devastating outbreak that is still continuing in West Africa, with approximately 50% mortality. It started in December 2013 from a single case of a two year old boy. The big difference with this new outbreak appears to be that it is occurring in large urban areas, rather than self-limiting small isolated villages. Possibly, humans are encroaching on more natural Ebola territory.
The Ebola virus first attacks macrophages and dendritic immune cells. These are the cells that pick up microbes and present them to T cells for stimulation of the immune response. By damaging early immune cells, Ebola becomes very effective. As the dendritic and macrophages die, they produce a cytokine storm–a desperate trigger of a large amount of signals–which further hurts immune cells, as well as other tissues. Ebola then attacks many other organs. Unlike HIV, Ebola is able to invade almost all other human cells, except for lymphocytes.
This post will describe what is known about the remarkable life style of Ebola.
Ebola’s Seven Genes
 Ebola virus does an incredible amount with its 7 genes. Unlike other viruses that rapidly mutate, such as HIV, the genetic material from each of Ebola’s five subtypes has been extremely stable over 40 years.
Ebola virus does an incredible amount with its 7 genes. Unlike other viruses that rapidly mutate, such as HIV, the genetic material from each of Ebola’s five subtypes has been extremely stable over 40 years.
The form of the single strand of RNA is called antisense, which means that it is not the type of RNA that can directly make a protein, but rather the complementary type that needs to first be copied in the cell to produce a messenger RNA. The messenger RNA manufactures new virus proteins. The polymerase protein needed for these copies is carried along with the RNA in the capsule.
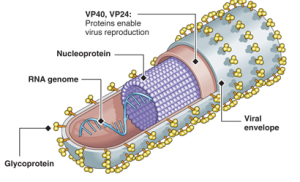
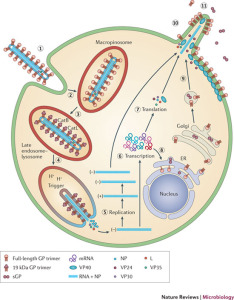
 The seven genes are NP-VP35-VP40-GP-VP30-VP24-L. The genetic material includes overlaps of genes. These seven genes make 8 proteins. NP stands for nucleoprotein and VP stands for virus protein. L is the polymerase molecule that copies the RNA.
The seven genes are NP-VP35-VP40-GP-VP30-VP24-L. The genetic material includes overlaps of genes. These seven genes make 8 proteins. NP stands for nucleoprotein and VP stands for virus protein. L is the polymerase molecule that copies the RNA.
One gene makes the RNA polymerase and another makes the glycoprotein for the envelope. A different gene makes the nucleoprotein that holds the genetic material. Separate genes make proteins VP40 and VP24 for the matrix. Other genes make proteins VP30 and VP35, which, along with the nucleoprotein, create a large complex with polymerase to manufacture more RNAs.
Ebola Virus Structure
 The Ebola virus has a long capsule with the appearance of a crooked, branching filament, surrounding a single small strand of RNA. The crookedness allows Ebola to evade antibodies and detection.
The Ebola virus has a long capsule with the appearance of a crooked, branching filament, surrounding a single small strand of RNA. The crookedness allows Ebola to evade antibodies and detection.
This large virus structure is composed of 3 layers—the nucleocapsid, the matrix space and the envelope. At the very center, inside the nucleocapsid and along the axis, is an empty space of 20 nm diameter. The virus shape is very variable with long tubes and many turns and branches. The long filaments are 80 nanometers in diameter and either 800 to 1000 nanometers long. RNA is only 1% of the mass of the virus, most of it being in these covering layers and the enzymes that are carried with it to invade the cell.
The three layers are:
 The nucleocapsid consists of virus proteins attached to the piece of RNA. The nucleocapsid is wound in a regular helix shape (50 nanometers in diameter). The major protein in the helix is NP with a smaller amount of two other two proteins, VP35 and polymerase (L). Later, they form a complex including VP30 to copy the virus. VP35 is essential in determining whether the polymerase will make the essential sense version of RNA to make proteins or the complementary version that travels with the virus.
The nucleocapsid consists of virus proteins attached to the piece of RNA. The nucleocapsid is wound in a regular helix shape (50 nanometers in diameter). The major protein in the helix is NP with a smaller amount of two other two proteins, VP35 and polymerase (L). Later, they form a complex including VP30 to copy the virus. VP35 is essential in determining whether the polymerase will make the essential sense version of RNA to make proteins or the complementary version that travels with the virus.- The next layer is the matrix space that is made up of mostly VP40 with some VP24. After the membrane is removed, these proteins help the virus enter into the cell by being attractive to cell membranes. They, also, manipulate the cell’s machinery and help with the final reassembly before it leaves the cell.
- The covering envelope is made of membrane created in the cell, with the regularly spaced GP spikes inserted while it is being manufactured in the endoplasmic reticulum and Golgi.
 The Ebola envelope has glycoproteins (GP) sticking through, which are the only target of antibodies. The trans membrane glycoprotein sticks out 7 nanometers from the lipid bilayer membrane at exact regular intervals and determines entry of Ebola into cells. The GP interacts with multiple factors on the surface of human cells. The special glycoprotein has many attached sugars and acetyl groups in the tail.
The Ebola envelope has glycoproteins (GP) sticking through, which are the only target of antibodies. The trans membrane glycoprotein sticks out 7 nanometers from the lipid bilayer membrane at exact regular intervals and determines entry of Ebola into cells. The GP interacts with multiple factors on the surface of human cells. The special glycoprotein has many attached sugars and acetyl groups in the tail.
Attaching to the Membrane
 Ebola first attaches with its glycoprotein protrusions to many different molecules on the cell’s membrane surface—lectins, integrins, cathepsins and others. There are, also, a large number of co factors that help Ebola to enter different types of human cells.
Ebola first attaches with its glycoprotein protrusions to many different molecules on the cell’s membrane surface—lectins, integrins, cathepsins and others. There are, also, a large number of co factors that help Ebola to enter different types of human cells.
Among the molecules that attach to Ebola, lectins focus the attachment. Cathepsins alter the virus glycans by removing the cap on the protrusion to help attachment. TAM enzymes (a family of receptor tyrosine kinases) allow entry into some cells. The protein NPC1 (Niemann-Pick C1 protein) works with the lysosome proteins and aids entry into all human cells. Fusion is stimulated by pH changes in special compartments.
Glycoprotein is manufactured in the secretory pathways described in the post on the complexity of membranes. The secretory pathway for lipoproteins and glycoproteins extends from the endoplasmic reticulum (ER) and the Golgi to the various membranes of the cell. In the ER and Golgi, the original protein is given attachments of sugars linked by oxygen or nitrogen.
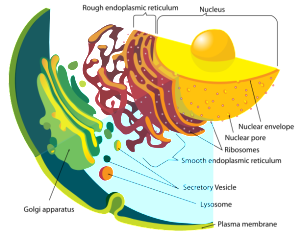 In the Golgi, the original precursor of GP is cut into two parts by a molecule called furin—these two connected subunits of the final structure are GP1 on the surface for contact and GP2 placed inside the membrane. The membrane with the GPs are then placed into part of the cell’s outer membrane, which is used to coat the final virus as it buds out of the cell. There are a large number of sugars attached to GP and also, a mucin region that is different in different strains.
In the Golgi, the original precursor of GP is cut into two parts by a molecule called furin—these two connected subunits of the final structure are GP1 on the surface for contact and GP2 placed inside the membrane. The membrane with the GPs are then placed into part of the cell’s outer membrane, which is used to coat the final virus as it buds out of the cell. There are a large number of sugars attached to GP and also, a mucin region that is different in different strains.
The surface GP, sticking out perpendicular to the surface of the virus membrane, is what creates the fusion with the cell membrane for entry. The host cell alters the virus GP, allowing it to attach. Part of this alteration is removing a cap on the perpendicular GP. The GP’s shape is dramatically altered in this process exposing a loop, which makes hairpin turns. The loop inserts into the cell membrane for a very strong attachment. Other folds occur and the two membranes come very close together and fuse.
There are many factors that aid this process. These different co factors aid Ebola in the intricacies of each type of human cell, not just the first macrophages that it encounters. Major targets are connective tissues and cells lining liver, kidney, lungs and other organs. Later it attacks the blood vessel cells. The only cells not available to Ebola are the lymphocytes, but it does decrease lymphocyte effectiveness.
The attachment process is very complex and involves proteins in the membrane and deep inside the cell. In different human cells, multiple varied factors stimulate many signaling cascades for fusion and lysosomal structures for removing the glycan cap.
Attachment factors all stick out of the cell’s membrane.
- Lectins allow Ebola to bind to sugars in different ways in many cell types: dendritic cells, alveolar macrophages, platelets, living cells of liver and lymph, bone marrow; and peripheral blood cells.
- TIM (T cell Ig mucin) is a mucin surface molecule, which is a receptor for other viruses such as hepatitis A. It is a membrane glycoprotein with specific binding sites. TIM helps Ebola bind to eye epithelium, kidney tubules, lung epithelia, and blood cells.
- Other types of factors bind in all cells: Axl, Cathepsin, and NPC1.
- Another set of unique factors affect the signaling from the membrane to the nucleus. TAMs are kinase enzymes that are very important in many cell functions: adhesion, reproduction, and cytokines. TAMs are very involved in Ebola entry into different cells.
- Integrins are very complex molecules with 24 different types based on subunits. They are unique to different Ebola entry as attachment factors.
Entering the Cell – Macropinocytosis
 Ebola has many different methods of attaching to the cell, which are tailored for the specific type of organs being invaded. But, they all result in fusion with the outer cell membrane through a unique type of engulfing process, called macropinocytosis. Macropinocytosis uses the cell’s membrane to make a very large sac in order to take large particles like Ebola inside the cell.
Ebola has many different methods of attaching to the cell, which are tailored for the specific type of organs being invaded. But, they all result in fusion with the outer cell membrane through a unique type of engulfing process, called macropinocytosis. Macropinocytosis uses the cell’s membrane to make a very large sac in order to take large particles like Ebola inside the cell.
Ebola’s glycoproteins are able to stimulate the unique rapid process of macropinocytosis, whereby the membrane creates large clefts, surrounds the virus and then brings it into the cell in a vesicle (sac). The process is unusual and different from the more widely known pinocytosis, which uses clathrin mechanisms similar to the membrane fusion that releases and re cycles neurotransmitters. Ebola is a very large virus, despite having only 7 genes, and because of this, the usual clathrin mechanisms, that create vesicles for small molecules, cannot be used. Mancropinocytosis is able to engulf very large particles like the Ebola virus.
 In order for macropinocytosis to work, Ebola first stimulates the cell’s programmed cell death pathway (apoptosis). But, it is a trick, because the cell death never occurs; Ebola needs to keep the cell alive. Macropinocytosis occurs normally as part of apoptosis. Ebola glycoproteins, along with the cell’s tyrosine kinase receptors, stimulate a particular type of lipid in the membrane tricking the cell to signal apoptosis. This creates the large vesicle to bring in the entire huge Ebola virus.
In order for macropinocytosis to work, Ebola first stimulates the cell’s programmed cell death pathway (apoptosis). But, it is a trick, because the cell death never occurs; Ebola needs to keep the cell alive. Macropinocytosis occurs normally as part of apoptosis. Ebola glycoproteins, along with the cell’s tyrosine kinase receptors, stimulate a particular type of lipid in the membrane tricking the cell to signal apoptosis. This creates the large vesicle to bring in the entire huge Ebola virus.
These special unusual large sacs are, then, taken to a unique lysosomal compartment of the cell that has low pH. The low pH is necessary to remove Ebola’s coat without hurting the rest of the virus. Lysosomes are large vesicle factories that have powerful enzymes to destroy debris and dangerous microbes.
Instead of killing Ebola, the lysosomes open up the vesicles and the virus envelope, releasing Ebola RNA and proteins into the cell. In this process, specific enzymes cut special glycoproteins. These enzymes cut out the section of the glycoprotein with many sugars and leave a region that is able to connect with a specific receptor on the lysosome and uncover and release the RNA.
Inside the Cell
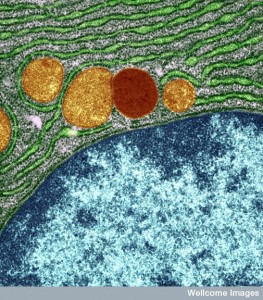 All Ebola replication is accomplished in the cytoplasm. Ebola doesn’t enter the nucleus. It uses the cell’s machinery of ribosomes and transfer RNA to make proteins from the viral messengerRNA.
All Ebola replication is accomplished in the cytoplasm. Ebola doesn’t enter the nucleus. It uses the cell’s machinery of ribosomes and transfer RNA to make proteins from the viral messengerRNA.
It is VP30 that triggers the start of manufacturing copies of the virus RNA. Phosphorus is attached and VP30 separates from the large complex holding the nucleoprotein. The RNA is copied and the new coat is constructed by VP24, VP30 and VP35.
Perhaps the most critical part of Ebola’s life is when it re assembles the new virus and escapes from the cell by budding through the membrane. VP40 has special ways to interact with the cell’s membrane. In fact, it can form its own vesicle and escape from the cell alone. But, with the help of GP and NP, the lipid raft in the membrane is manipulated to form the large special structure that assembles the virus before it buds from the cell.
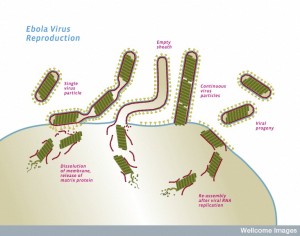 While the virus is being constructed, first the critical polymerase protein connects with the ribonucleoprotein through action of VP35. The proteins attached to the Ebola RNA then connect with the matrix proteins. Once this complex is assembled, the entire structure focuses on a section of the membrane lipid raft (see previous post on membranes).
While the virus is being constructed, first the critical polymerase protein connects with the ribonucleoprotein through action of VP35. The proteins attached to the Ebola RNA then connect with the matrix proteins. Once this complex is assembled, the entire structure focuses on a section of the membrane lipid raft (see previous post on membranes).
The lipid raft is a highly organized complex structure floating in the membrane. The lipid raft builds the long membrane sheath with the GP sticking out, which at first is empty. The assembled inner layers of the virus move into the empty membrane sheath, which is waiting for them. Then, the entire new virus buds off from the cell’s membrane.
Tetherin is a molecule stimulated by interferon that stops the virus from budding, as well as causing other disruptions in Ebola’s process. HIV is able to stop the effects of tetherin. Ebola, in a similar way, stops Tetherin using its trans membrane glycoprotein. The GP uses multiple mechanisms for this, including blocking Tetherin from the sections of the membrane that it uses to bud.
VP35 Protein Helps Fight the Immune System
 VP35 is a key factor that connects polymerase with the nucleo protein. Just as critical, it inhibits alpha and beta interferon in many different ways. Interferons are the key cytokines used by the immune system to fight virus infection.
VP35 is a key factor that connects polymerase with the nucleo protein. Just as critical, it inhibits alpha and beta interferon in many different ways. Interferons are the key cytokines used by the immune system to fight virus infection.
Several pattern recognition receptors in the cell notice there is RNA in the cytoplasm. These receptors signal the powerful cytokine NF-kB, and several other interferons that cause inflammation to fight the virus.
VP35 is able to disrupt these immune cytokine pathways preventing addition of phosphorus onto critical molecules in the recognition cascade. It, also, acts as a decoy in another process, and interferes with protein interactions that would help the immune response. It is amazing that one protein can do all of this. VP35, also, has a direct interaction with RNA to prevent its attachment to receptors. Studies comparing the Reston (Virginia) Ebola of monkeys and the Zaire show that there are other unknown factors helping this disruption of the immune response.
Another way that VP35 interacts is through the SUMO system. Studies have identified multiple ways VP35 disrupts SUMO systems preventing immune responses. (See post on ubuiquitin and SUMO, the cell’s major molecular tagging systems)
The result of all of these different mechanisms is to prevent interferon responses that would otherwise kill the virus.
VP24 Protein Also Fights the Immune System
 The first immune response against a virus is interferon, which signals all the cells to fight, including using antibodies. This occurs by interferon receptors creating a phosphorylation cascade, stimulating activators to the nucleus, triggering genes that fight the virus. Ebola blocks the production of interferon. But, it also stops the cell’s responses to all types of interferon.
The first immune response against a virus is interferon, which signals all the cells to fight, including using antibodies. This occurs by interferon receptors creating a phosphorylation cascade, stimulating activators to the nucleus, triggering genes that fight the virus. Ebola blocks the production of interferon. But, it also stops the cell’s responses to all types of interferon.
VP24 both blocks the signals and the nuclear activators in this process. Activators are transported to the nucleus in a complex system to signal more immune activity. VP24 binds to this complex blocking it. VP24 does this without stopping other necessary travel to the nucleus, keeping the cell alive and able to be used by Ebola. VP24 demonstrates multiple mechanisms blocking interferon α, β, and γ.
VP24, along with the virus nucleoprotein, is very important to the deadly effectiveness of Ebola. VP24 and VP35 both disrupt multiple kinds of interferon, but, also, stop the responses to interferon.
Taking Over Cell Functions and Evasion of Immune Functions
When a virus enters a cell, the cell decreases production of proteins through a kinase reaction to block creation of new viruses. VP35 blocks this cell reaction and instead stimulates increased protein production. Ebola, with only seven genes, is able to manipulate many complex cellular processes.
Cell Nuclear Process Altered
 Normally, mechanisms inside the nucleus have complex relations with messenger RNA and the alternative RNA editing process (see RNA alternative editing post). Specific protein complexes help splice the messengerRNA before it is sent from the nucleus to the ribosomes in the cytoplasm to make proteins. Ebola VP24 manipulates this process and, somehow, takes it out of the nucleus allowing it to increase Ebola production in the cytoplasm.
Normally, mechanisms inside the nucleus have complex relations with messenger RNA and the alternative RNA editing process (see RNA alternative editing post). Specific protein complexes help splice the messengerRNA before it is sent from the nucleus to the ribosomes in the cytoplasm to make proteins. Ebola VP24 manipulates this process and, somehow, takes it out of the nucleus allowing it to increase Ebola production in the cytoplasm.
Ebola’s Response to Cell RNAi Interference
When RNA viruses enter cells, special interference RNAis are manufactured by the cell. This process cuts viral RNA into small pieces that interfere with its own function; this RISC mechanism is the internal cellular immune system (see post). VP35 interacts with the vital RISC complex stopping the process. This eliminates a major mechanism for the cell to fight viruses. Another Ebola protein, VP30, adds yet another mechanism in silencing RISC, stopping RNA inference altogether.
Thus, Ebola uses two different proteins to interrupt the cell’s protective RNAi immune reaction.
Ebola Triggers Strong Immune Reactions, Which Can Help It Survive
 When Ebola stimulates the immune system, this can either help the host’s survival or help Ebola, causing human death. Those people who survive Ebola have larger amounts of specific varieties of T cells, especially cytotoxic T cells.
When Ebola stimulates the immune system, this can either help the host’s survival or help Ebola, causing human death. Those people who survive Ebola have larger amounts of specific varieties of T cells, especially cytotoxic T cells.
Ebola virus is first taken up by dendritic cells and macrophages, which then bring it to the lymph and bodily tissues. Usually, viruses are brought there to present the virus molecules to T cells, which stimulates fighting cells and antibodies. Ebola doesn’t allow this T cell process to occur. Instead, it is able to reduce the amount of important cytokine signaling and increase others types of signals, including altering TNF-alpha, Il-6 and Il-10. These are correlated with human mortality from Ebola.
Also, the dendritic cells carrying Ebola become deficient in that they don’t stimulate the usual CD4T cells to fight the infection. Most recent studies show that people who can overcome the disruptions caused by VP24, VP30 and VP35 survive.
With only 7 genes, Ebola has many ways to avoid and fight antibodies. When Ebola virus is able to kill blood cells, then there aren’t many antibodies produced. The large size of the virus filaments, which are filled with stable glycoproteins, is a big obstacle for antibodies. The long filament structure folds and creates pockets that antibodies are not able to reach. Many attached sugars, also, make it inaccessible to antibodies. The unusual rapid process of macropinocytosis stops the effects of antibodies. Another problem with antibodies is that the GP cap covers the molecules that would be attacked by antibodies. A special lysosome process removes the cap and only then is Ebola susceptible to antibodies. But, by then Ebola is already copying itself.
Ebola’s Glycoprotein Decoy
 Ebola virus uses another remarkable mechanism where it creates a decoy for the immune system to attack instead of the virus. The Ebola gene makes two closely related versions of its glycoproteins. One is used for the covering of the virus. The other, sGP, is a large protein used as a decoy to the immune system.
Ebola virus uses another remarkable mechanism where it creates a decoy for the immune system to attack instead of the virus. The Ebola gene makes two closely related versions of its glycoproteins. One is used for the covering of the virus. The other, sGP, is a large protein used as a decoy to the immune system.
Ebola has a mechanism to mix the production of these two, where polymerase moves back and forth in an unusual way—first making one then the other. In fact, 80% of the glycoproteins are decoys that evade the immune responses. The process where both types are made is, somehow, highly regulated by Ebola. Production of new viruses is balanced with the need to evade the attack of the immune system. sGP, in fact, is not only a decoy, but actively disrupts the response of white blood cells in their attack.
The Very Intelligent Ebola Virus Takes Front and Center

While there is much more to be learned about Ebola, it is clear that this virus has a very complex lifestyle.
Using only seven genes, Ebola’s small piece of RNA builds a very large, complex vessel for travel. Ebola is able to use many varied factors to attach to each type of human cell. It tricks the cell in two ways—first, to stimulate a very unusual entry for this large structure, and second to create a decoy that the immune system attacks instead of the virus. It takes over the cell’s genetic manufacturing and evades immune attacks in many different ways. It builds new viruses by manipulating special complex rafts in the cell’s membrane.
How is this possible? How can one small piece of RNA orchestrate all of these maneuvers and intelligent evasion techniques? Where is the direction for all of this?
The direction for the entire process can’t be in one small piece of RNA. Or can it? Does mind and intelligence, somehow, interact with these molecules?