 A previous post noted how microbes can help cancers in all stages of their development. Now, it has been found that the one-time microbe now the mitochondria is also vital for cancer to start, to grow, to survive and to metastasize. These microbes and the mitochondria use back and forth communication to help cancers in many ways. This post describes the recent research about mitochondria and its vital relationship to cancer.
A previous post noted how microbes can help cancers in all stages of their development. Now, it has been found that the one-time microbe now the mitochondria is also vital for cancer to start, to grow, to survive and to metastasize. These microbes and the mitochondria use back and forth communication to help cancers in many ways. This post describes the recent research about mitochondria and its vital relationship to cancer.
Mitochondria Joined Forces With Our Cells
Two billion years ago approximately, the microbe ancestor of our organelle mitochondria chose to live a safer life inside of our larger cell. Accommodations were made over a long period of time to share functions with the big cell. Much of the mitochondria’s genome was dismantled and sent to the nucleus of the large cell or the cell took over those functions with their own genes. However, the mitochondria maintained its vital energy production activity with all of the hugely complex molecules for electron transfer and respiration cycles and the complex genes and enzymes involved. Elaborate communication between the mitochondria and the larger cell has continued and contributes to all vital activity maintaining the cell.
 Mitochondria produce energy and molecules and converse about many issues with the larger cell, but particularly about stress and how the cell deals with it. Because of their importance in dealing with stressful events for the cell, mitochondria are vital parts of pathways that can help or hurt the creation of cancers. Pathways that are vital to both mitochondria and cancer include producing more mitochondria, the ways that allow mitochondria to break into two and to fuse together, triggers for programmed cell death, regulation of reactive oxygen species (ROS) that lead to DNA breaks and mutations, general metabolism, and conversations among organelles inside the cell.
Mitochondria produce energy and molecules and converse about many issues with the larger cell, but particularly about stress and how the cell deals with it. Because of their importance in dealing with stressful events for the cell, mitochondria are vital parts of pathways that can help or hurt the creation of cancers. Pathways that are vital to both mitochondria and cancer include producing more mitochondria, the ways that allow mitochondria to break into two and to fuse together, triggers for programmed cell death, regulation of reactive oxygen species (ROS) that lead to DNA breaks and mutations, general metabolism, and conversations among organelles inside the cell.
Mitochondria were discovered around 1890 and by 1913 Warburg stated they were involved in transfer of oxygen and production of energy. Later the TCA metabolic cycle was found and processes to generate usable electrons for respiration and the transport mechanisms of electrons were found. Warburg also discovered that cancers use a different metabolism (lactate instead of glucose) when oxygen was present and it is now called the Warburg effect. He stated that damaged mitochondria helped cancers. Recently, this understanding has expanded to find that mitochondria can be functioning and helping the cancers in many other ways.
 Mitochondria are known to make the vital energy particle ATP. but they do much more including sensing stress by regulating ROS, cell death, and metabolism. They help cancers survive in difficult environments with little food and oxygen and during cancer treatments. As all aspects of cancer have been found to be very varied and complex, so also do mitochondria have many different ways of functioning with cancers, both harmful and helpful.
Mitochondria are known to make the vital energy particle ATP. but they do much more including sensing stress by regulating ROS, cell death, and metabolism. They help cancers survive in difficult environments with little food and oxygen and during cancer treatments. As all aspects of cancer have been found to be very varied and complex, so also do mitochondria have many different ways of functioning with cancers, both harmful and helpful.
The Life Cycle of A Cancer and Mitochondria
 The most recent research shows that mitochondria help cancers grow in all of their multiple stages—initiation, growth, survival, and maintenance. Sometimes cancers can begin their development when stimulated by particular metabolites produced by enzmes in mitochondria. These are now called oncometabolites. Another important stimulus for the start of cancers is stress from oxidation pathways that create stress on the entire function of the cell and therefore stimulate new and abnormal pathways to deal with the stress. Other factors for initiation of the cancer are signals from the mitochondria to the cell.
The most recent research shows that mitochondria help cancers grow in all of their multiple stages—initiation, growth, survival, and maintenance. Sometimes cancers can begin their development when stimulated by particular metabolites produced by enzmes in mitochondria. These are now called oncometabolites. Another important stimulus for the start of cancers is stress from oxidation pathways that create stress on the entire function of the cell and therefore stimulate new and abnormal pathways to deal with the stress. Other factors for initiation of the cancer are signals from the mitochondria to the cell.
The next phases of cancer are growth and survival. The cancer must grow more than other cells to create an entirely new society of cells—an entire new autonomous bodily organ that is supported by the usual connective local cells and blood vessels. When stress alters the way the mitochondria function, new metabolic pathways are programmed and new signals are sent from the mitochondria to promote the cancer cell and help with survival. Mitochondria change their shapes in response to the oxidative stress and the regulation of metabolism called redox.
 Another important factor is the pathway to cell death that is usually a brake on creating very abnormal types of cells. The mitochondria is instrumental in letting the cell know that something is awry, that there is not enough energy, that there are too many mutations, and to signal death of one cell to save the organ and the creature. When the mitochondria alter their approach to cell death this helps the cancers survival. The shape alterations and the process of creating new mitochondria also help the cell survival.
Another important factor is the pathway to cell death that is usually a brake on creating very abnormal types of cells. The mitochondria is instrumental in letting the cell know that something is awry, that there is not enough energy, that there are too many mutations, and to signal death of one cell to save the organ and the creature. When the mitochondria alter their approach to cell death this helps the cancers survival. The shape alterations and the process of creating new mitochondria also help the cell survival.
For metastasis, the mitochondria must make many new energy producing comrades and launch them to new sites. Also, when mitochondria are reprogrammed they produce a very different metabolism that helps the cell travel and produce a new metastatic community.
Mitochondria Helping Produce Tumors in Stages
 Mutations and DNA breaks can occur in the genes inside of the mitochondria as well as the cell at large. Unusual metabolic products produced by mutated mitochondrial genes help the cancer (oncometabolites.) Other factors include the changes that occur with stress of the mitochondrial function, particularly too many oxygen products. Mitochondria are responsible to regulate the amount of oxygen reactions (called redox). This regulation plays into whether a cell will decide to survive or stimulate pathways of programmed cell death. The number of available mitochondria is part of this picture, with a great number helping the exponential growth of the tumor cells. Mitochondria can be reprogrammed as well in this process. Signals from mitochondria to the cell can stimulate tumor growth.
Mutations and DNA breaks can occur in the genes inside of the mitochondria as well as the cell at large. Unusual metabolic products produced by mutated mitochondrial genes help the cancer (oncometabolites.) Other factors include the changes that occur with stress of the mitochondrial function, particularly too many oxygen products. Mitochondria are responsible to regulate the amount of oxygen reactions (called redox). This regulation plays into whether a cell will decide to survive or stimulate pathways of programmed cell death. The number of available mitochondria is part of this picture, with a great number helping the exponential growth of the tumor cells. Mitochondria can be reprogrammed as well in this process. Signals from mitochondria to the cell can stimulate tumor growth.
Some specific pathway changes also allow the ability of the cell to bypass the normal metabolic cycles, such that metastatic states can arise. The ability of the cell to alter its metabolism in unusual ways helps cancers survive and grow.
The opposite processes are important also. The normal process of destruction of defective or unnecessary mitochondria can be altered to produce too many.
 A general increase in production of all mitochondrial proteins moves in the direction of cancers. Special factors have been identified that help create cancers form these abnormal pathways when there is a great increase in protein production in the mitochondria. One particular transcription factor is well known (c-Myc) that is very important in the cycles of cell reproduction, growth, metabolism, and programmed cell death. Myc targets at least 400 different genes in the mitochondria. More Myc, the more mitochondria; decreased Myc slows them down. In the normal cell Myc creates an interaction with the cycle that produces cell division with production of more mitochondria to make a new cell.
A general increase in production of all mitochondrial proteins moves in the direction of cancers. Special factors have been identified that help create cancers form these abnormal pathways when there is a great increase in protein production in the mitochondria. One particular transcription factor is well known (c-Myc) that is very important in the cycles of cell reproduction, growth, metabolism, and programmed cell death. Myc targets at least 400 different genes in the mitochondria. More Myc, the more mitochondria; decreased Myc slows them down. In the normal cell Myc creates an interaction with the cycle that produces cell division with production of more mitochondria to make a new cell.
mTOR is another important molecule that also increases the number of mitochondria. mTOR regulates ribosomes, protein construction, and the amounts of amino acid precursors. It is very involved in regulating energy with mitochondria. In cancer, mTOR activity can be abnormal. It affects mitochondria by altering both genetic and protein methods. For example, decreasing necessary proteins for mitochondria.
By controlling these genetic and protein networks, cancers can adapt to fight against treatments and work to get the supporting neighborhood cells to help. By altering mitochondria both pancreatic cancer and melanoma are more successful at fighting treatments. One targets metformin, while maintaining great mitochondrial activity.
More has to be understood about how mitochondria can alter their functions to help cancer.
Killing Mitochondria
 When mitochondria are damaged, they are either removed by quality control, or fuse with healthy mitochondria. When the machinery that moves electrons is impaired it produces reactive oxygen species (ROS), which are molecules that can cause mutations and increase cancer’s activity. Also, low oxygen alters this function. The well-known Bcl-2 pathway affects both death of mitochondria and protection against cancer.
When mitochondria are damaged, they are either removed by quality control, or fuse with healthy mitochondria. When the machinery that moves electrons is impaired it produces reactive oxygen species (ROS), which are molecules that can cause mutations and increase cancer’s activity. Also, low oxygen alters this function. The well-known Bcl-2 pathway affects both death of mitochondria and protection against cancer.
Pathways of programmed cell death can both help and hurt cancers. This is similar to the process of autophagy (a process whereby cells break down debris in a programmed way) that is helpful and harmful to cancers. It appears to depend upon the stage of development. This was also noted in a previous post on the ways that microbes help and hurt cancers.
By inhibiting the natural killing of mitochondria, problematic mitochondria survive and increase ROS and mutations that are good for cancers. But, when the cancer is established, then defective mitochondria can hurt the cancer and need to be eliminated for survival. In one type of cancer the more abnormal mitochondria allowed to survive the less malignant the cancer is.
Previous posts described the elaborate communication between mitochondria and endoplasmic reticulum, their home base in communication with the cell, about the dynamic activity of breaking into more mitochondria (fission) and combining to form larger but less numbers of mitochondria (fusion). The membrane fission and fusion dynamics are complex with many proteins involved. This process is influenced by addition of phosphorus tags, by cell stress, and by the stage of cell division, where more mitochondria are needed for cell division. Mitochondria can be long and networked or short and granular.
These processes are abnormal in cancer—often more fission and or decreased fusion at first. This causes more breakup of the network. This leads to more ROS and more mutations. This occurs by influencing multiple proteins in many different ways. But, later in the cycle remodeling produces more fusion. One identified process involving c-Myc leads to more fusion.
Cell Death Pathways to Help Destroy Defective Cells
 Cancer cells avoid dying in multiple ways and often with the help of mitochondria. The protective Bcl-2 pathway leads to programmed cell death when that is the only solution for a problem cell that can harm the entire organ. In this complex pathway several molecules help the process of fission and fusion of mitochondria. These create holes in the membranes and trigger caspase enzymes that lead to cell death. Bcl-2 can be manipulated by cancers to actually works against programed cell death and stop these molecules from causing membrane holes in the mitochondria. Cancers trigger gene networks that stop the cell death activity and increase those that do the opposite. Where the cancer sits in this continuum in the pathway determines how it will survive attack from cancer treatments.
Cancer cells avoid dying in multiple ways and often with the help of mitochondria. The protective Bcl-2 pathway leads to programmed cell death when that is the only solution for a problem cell that can harm the entire organ. In this complex pathway several molecules help the process of fission and fusion of mitochondria. These create holes in the membranes and trigger caspase enzymes that lead to cell death. Bcl-2 can be manipulated by cancers to actually works against programed cell death and stop these molecules from causing membrane holes in the mitochondria. Cancers trigger gene networks that stop the cell death activity and increase those that do the opposite. Where the cancer sits in this continuum in the pathway determines how it will survive attack from cancer treatments.
The shapes of mitochondria are vital in this entire process. Very disorganized mitochondria networks (fragmented) cause less programmed death pathways. With a more organized network, cell death operates again. It is not that the shape causes cell death or not, but that it works with and interacts with the Bcl pathway molecules for these effects.
Cell Stress and Cancer
 Several oxygen activities produce highly reactive ROS. Hydrogen peroxide is one oxygen reactive species and superoxide and free radicals of the hydroxyl molecules are another. These are naturally produced as part of the machinery of mitochondria that produces energy for the cell. Mitochondria have many specific pathways to inhibit the negative effects of these over-active oxygen pathways. These include the molecules enzyme superoxide dismutase (SOD2), peroxidoxins, thioredoxin, and glutathione.
Several oxygen activities produce highly reactive ROS. Hydrogen peroxide is one oxygen reactive species and superoxide and free radicals of the hydroxyl molecules are another. These are naturally produced as part of the machinery of mitochondria that produces energy for the cell. Mitochondria have many specific pathways to inhibit the negative effects of these over-active oxygen pathways. These include the molecules enzyme superoxide dismutase (SOD2), peroxidoxins, thioredoxin, and glutathione.
At first it was thought that just stopping ROS helped avoid cancer. But it is more complex than that. ROS increases cell growth and communication of the cells. Strangely, the more strongly the pathways against ROS increase, the less there is cell death of the cancer cell itself. Therefore, the opposite can occur, that is, more cancer.
The mitochondria reactions that produce ROS are very complex and increased by cancers and their signaling communication in the special environments of cancers without oxygen. Also mutations can occur in the large proteins of the mitochondria machinery. ROS affects large molecules of protein, DNA, and lipids.
 One of the effects is stopping a protein that normally suppresses cancers (PTEN) by altering cysteine. It affects many enzymes in ways that are not yet understood (phosphatases) and affect mitochondrial death. These affect signals from the cancers causing more metastasis signaling. In this case eating ROS helped stop these metastases.
One of the effects is stopping a protein that normally suppresses cancers (PTEN) by altering cysteine. It affects many enzymes in ways that are not yet understood (phosphatases) and affect mitochondrial death. These affect signals from the cancers causing more metastasis signaling. In this case eating ROS helped stop these metastases.
Surprisingly, many cancers increase pathways against ROS. These can stop the metastasis, but help survival of the individual initial cancer. But, in another situation both the cancer becomes more malignant and increases metastasis. It appears that cancers like just enough ROS but not too much. In this window there is more cancer growth, but less cell death. Just the right amount of treatments that stop ROS have been helpful, also, as have targets of other mitochondria pathways. This means finding the sweet spot in the treatment, where too much or too little won’t help.
Altered Metabolism
 A previous post noted how cancer alters their own cellular metabolism, in the same ways that T cells alter their metabolism to change and become killer cells. This occurs by using normal metabolic pathway molecules as signals to genes. Changes in cancers increase production of large molecules, needed for energy, and decrease of cell death.
A previous post noted how cancer alters their own cellular metabolism, in the same ways that T cells alter their metabolism to change and become killer cells. This occurs by using normal metabolic pathway molecules as signals to genes. Changes in cancers increase production of large molecules, needed for energy, and decrease of cell death.
Sugar pathways are altered and molecules instead enter pathways related to amino acids, pentose, and lipids. These are instead of the usual mitochondrial respiration pathway. Pyruvate is diverted by a different cancer enzyme and molecules accumulate that build instead of breaking down molecules. These alterations change survival of cancers. Mitochondria alter their activity related to amino acids and fats.
Altering Amino Acid Pathways
 Altered metabolic pathways use glutamine for energy rather than sugar as well as molecule synthesis pathways. Alterations in these pathways increases production of some tumors. Mitochondria alterations are complex but help produce more energy for cancers in unusual situations and produce more cancer cell reproduction. Mitochondria adapt by producing cycles whereby electrons are removed from carbon at a greater rate as food is oxidized to make molecules for the cancers. They then have to use other pathways to deal with this increase of electrons. They use these to make more molecular parts of DNA (nucleotides) that help cancers grow and reproduce.
Altered metabolic pathways use glutamine for energy rather than sugar as well as molecule synthesis pathways. Alterations in these pathways increases production of some tumors. Mitochondria alterations are complex but help produce more energy for cancers in unusual situations and produce more cancer cell reproduction. Mitochondria adapt by producing cycles whereby electrons are removed from carbon at a greater rate as food is oxidized to make molecules for the cancers. They then have to use other pathways to deal with this increase of electrons. They use these to make more molecular parts of DNA (nucleotides) that help cancers grow and reproduce.
Fat metabolism is altered also, but in different ways. Different cancers seem to take different approaches. Some increase oxidation of fats. Others increase fat manufacture, which helps make more membranes for new cells. The mitochondria accommodate tumors by making more oxidized fats that are used as food. This is preferred fuel for distressed cancer cells. Stress is placed on cancer cells as they break from the extra cellular structures that cells normally live in.
This is all so complex, that new research needs to study cancer in real life situations, which is difficult.
Mitochondria Signals
 Mitochondria are in constant communication with the cell through contact with the endoplasmic reticulum and messages to the nucleus. The pathways in cancers change mitochondria to increase the growth of the cancer. Signals from the mitochondria affect the cancer in both directions. Changes (mutations) in mitochondria lead to unique new metabolisms for cancers to grow.
Mitochondria are in constant communication with the cell through contact with the endoplasmic reticulum and messages to the nucleus. The pathways in cancers change mitochondria to increase the growth of the cancer. Signals from the mitochondria affect the cancer in both directions. Changes (mutations) in mitochondria lead to unique new metabolisms for cancers to grow.
Those well-known proteins that suppress tumors and the genes that produce them (oncogenes) are vital to regulate mitochondria activity. Several notable pathways have been found to affect both mitochondria and cancers.
c-Myc was mentioned already to produce new mitochondria and help cancers. This pathway triggers genetic networks that increase the general production of RNA and proteins. It also is very involved in the unique alternative metabolic pathway described above for glutamine.
As well as pathways leading to more cell reproduction, cell death, and metabolism cancers are able to promote a surprisingly elaborate coordinated system to alter mitochondria in multiple ways at the same time.
 One alteration changes the normal respiration pathways and produces more fission of mitochondria. It alters mitochondria ability to survive when the cell is starving for food. By stopping autophagy, the process that breaks up material to be recycled, it allows survival without food for the mitochondria and therefore survival of the cancer.
One alteration changes the normal respiration pathways and produces more fission of mitochondria. It alters mitochondria ability to survive when the cell is starving for food. By stopping autophagy, the process that breaks up material to be recycled, it allows survival without food for the mitochondria and therefore survival of the cancer.
With low energy and starvation situations, common for cancers as the immune cells and medications try to kill them, mTOR does more than produce more mitochondria, it produces many different pathways, for example the little used folate pathway to help create purines for the cell.
Other pathways work with mTOR to triggers other alternative energy pathways. The results are different at the beginning of the tumor than after established cancer development. During severe starvation another pathway kills some of the mitochondria. But, if the starvation is long lasting then it will start stimulating new mitochondria for the cancer to continue to evolve.
p53 Pathway and Cancer
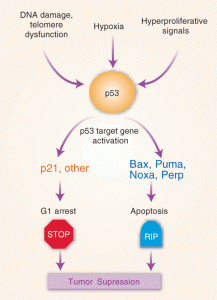 p53 is an important suppressor of cancers often altered by mutations. It is very interactive with the pathways of cell division and programmed cell death. It also stimulates particular genes that alter metabolism. In fact, it stimulates genes that produce the complex electron transfer machinery of the mitochondria and those that keep it going.
p53 is an important suppressor of cancers often altered by mutations. It is very interactive with the pathways of cell division and programmed cell death. It also stimulates particular genes that alter metabolism. In fact, it stimulates genes that produce the complex electron transfer machinery of the mitochondria and those that keep it going.
But, it can help the cancer survive by helping adaptation to stress. It can help with the adaptation to starvation by further altering metabolism as well. But, in this situation mutated p53 versions do not aid Bcl-2 as they usually do with programmed cell death and don’t allow holes to be cut in the outer membranes of mitochondria for the death process.
Signals from Mitochondria about Stress
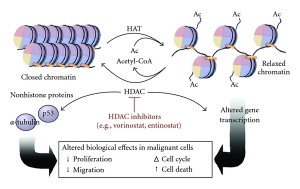 Mitochondria sense stress and send special signals to the cell. Signals are sent from the changing metabolic cycles to the nucleus through pathways that alter chromatin with tags of acetyl on histones (epigenetic mechanisms) and other regions of demethylation. Another citrate signal affects the enzyme histone acetyl transferase (HAT). These metabolic by products also signal and alter important proteins. They alter signal pathways, genetic stimulation of RNA, and epigenetic signals.
Mitochondria sense stress and send special signals to the cell. Signals are sent from the changing metabolic cycles to the nucleus through pathways that alter chromatin with tags of acetyl on histones (epigenetic mechanisms) and other regions of demethylation. Another citrate signal affects the enzyme histone acetyl transferase (HAT). These metabolic by products also signal and alter important proteins. They alter signal pathways, genetic stimulation of RNA, and epigenetic signals.
When the membrane characteristics of mitochondria are altered, they affect protein production. Also changes in the way electron transfers occur produce signals that produce these changes. ROS also signals to increase cancers.
Mitochondria Metabolites Spur Cancer
 When enzymes are altered by mutations, mitochondria signals spur oncometabolites. These occur in 70% of glioblastoma brain cancers, and 20% of myeloid leukemias. They complete for enzymes and alter important functions. They also affect histone epigenetic changes. The metabolites from mitochondria are very powerful at increasing cancer through these epigenetic stimuli.
When enzymes are altered by mutations, mitochondria signals spur oncometabolites. These occur in 70% of glioblastoma brain cancers, and 20% of myeloid leukemias. They complete for enzymes and alter important functions. They also affect histone epigenetic changes. The metabolites from mitochondria are very powerful at increasing cancer through these epigenetic stimuli.
Mitochondria have multiple copies of their genes in each in order to rapidly produce their proteins. There are many changes related to these DNA loops that help cancers. These three dimensional structural changes can alter the electron transfer producing oxidative stress and ROS.
Mitochondria Help Cancers Grow
 Mitochondria are very complex as is their interaction with the cell. They are involved in all aspects of the growth of cancers. They are very flexible and change at the different stages of the cancer including changes in energy, in use of fuel, in whether they will kill themselves or put up with all the mutations, and in dealing with oxidative stress and ROS.
Mitochondria are very complex as is their interaction with the cell. They are involved in all aspects of the growth of cancers. They are very flexible and change at the different stages of the cancer including changes in energy, in use of fuel, in whether they will kill themselves or put up with all the mutations, and in dealing with oxidative stress and ROS.
Mitochondria help the cancer during times of starvation, which is very important during cancer’s survival during treatments. For future treatments, these adjustments made from mitochondria must be understood and considered. The ways mitochondria talk with the cell are in some ways similar to signals among immune cells and bacteria. As these conversations are understood, new treatments will emerg.