 Each cell has oscillating gene networks that somehow help organize, synchronize, and anticipate activity of the tissues and the entire organism. Energy from the sun is transformed into energy and material for the cell to use in sync to these rhythms. The rhythms also are related to how the cell develops in particular organs and responses to damage and distress. It is not yet clear how these individual unique 24 hour clocks in each cell translates to the rhythms of the entire animal. In evolution, the development of these clocks appears to be vital to provide the needed resources for DNA repair at the proper time of day. Also, it provides machinery at the right time to make oxygen and a way to avoid expending energy for little gain.
Each cell has oscillating gene networks that somehow help organize, synchronize, and anticipate activity of the tissues and the entire organism. Energy from the sun is transformed into energy and material for the cell to use in sync to these rhythms. The rhythms also are related to how the cell develops in particular organs and responses to damage and distress. It is not yet clear how these individual unique 24 hour clocks in each cell translates to the rhythms of the entire animal. In evolution, the development of these clocks appears to be vital to provide the needed resources for DNA repair at the proper time of day. Also, it provides machinery at the right time to make oxygen and a way to avoid expending energy for little gain.
The two previous posts have described the discovery of individual clocks in each cell and the way individual cells interact with tissues and the brain clocks to regulate metabolism in the most efficient manner. This post describes the unique clocks in immune cells and how vital this is the response to infection and trauma. The next post will describe the central brain clocks that synchronize some of the circadian rhythms.
Immune Cell Clocks Among Other Rhythms
 Immune cells have their own unique clock and interactions with other clocks. Immune factors have daily rhythms and immune cells respond differently at different times of day. With these 24 hour clocks, individual cells are able to anticipate different environmental situations and respond appropriately. It is not yet clear how different larger rhythms in the organism interact—sleep, movement, temperature, eating, metabolic, and immune.
Immune cells have their own unique clock and interactions with other clocks. Immune factors have daily rhythms and immune cells respond differently at different times of day. With these 24 hour clocks, individual cells are able to anticipate different environmental situations and respond appropriately. It is not yet clear how different larger rhythms in the organism interact—sleep, movement, temperature, eating, metabolic, and immune.
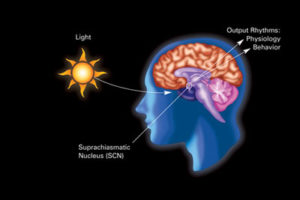
The central pacemaker in the suprachiasmatic nucleus brain center SCN has an effect on every cell’s clock. The SCN cycle is directly influenced by daylight and darkness of night. SCN has many independent interneurons that can maintain the cycles and send out the cycle to any region of the body. These SCN signals maintain many of the tissues. They maintain signals through the sympathetic nervous system to regulate temperature throughout the body and also rhythmically stimulate glucocorticoids that provide many specific metabolic changes. Alterations in these can lead to disease and some diseases reflect these rhythms—asthma, arthritis, and heart attacks.
These daily differences also occur with infections. Cycles affect production of white blood cells and innate and adaptive responses to particular microbe attacks. Increases responses in mammals appear to geared to particular times of day when looking for food.
Immune Cell Clocks
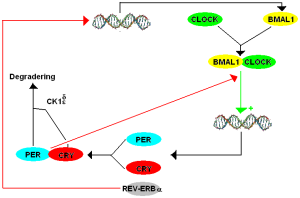
The core clock molecules are the same as in other cells, but with some differences (CLOCK/BMAL1, PER, REV-ERB, etc). (see previous post on individual cell clocks). There are two other circuits that work with the core loops to produce immune 24 hour cycles (including ROR and Albumin D-box with ilterleukin3). These feedback loops relate the special immune cycles to cellular metabolic cycles. Special cycles with oscillation signals are necessary to produce particular immune cells, such as natural killer cells and specific helper cells. Many of these cycles determine the triggering of particular cytokines such as CD4+ T helper cell secreting interleukin 17 (Th17). Local resident microbes interact in a rhythmic manner with the lining cells through pattern recognition receptors.
A positive immune response is necessary to fight microbes and to work on tissue repair from trauma. When the immune response is disrupted, illness can occur, such as the very serious illness of septic shock. In general, there are pluses and minuses of immune reactions. The down side includes using up energy and other resources and inadvertent damage to tissues. Another cost is being unprepared for infections that occasionally leads to deadly septic shock. In order to decrease the costs, the immune response can be dampened, or it can be used less frequently, such as only at times when the most danger exists. In evolution, timing is set based on the anticipated dangers.
 One mechanism to reduce costs is to limit some of the immune responses to particular times of day, eliminating random spontaneous activation. What makes this complex is that the various mechanisms of defense are at different times in the cycle—defense at lining cells, secretion of peptides against microbes, activation of complement, production of special immune cells, and particular cytokines. Each cell is produced differently—dendritic cells, macrophages, neutrophils and monocytes. When each of these factors are not at their strongest at the same time, they are together less likely to produce the catastrophic sepsis that needs all of them.
One mechanism to reduce costs is to limit some of the immune responses to particular times of day, eliminating random spontaneous activation. What makes this complex is that the various mechanisms of defense are at different times in the cycle—defense at lining cells, secretion of peptides against microbes, activation of complement, production of special immune cells, and particular cytokines. Each cell is produced differently—dendritic cells, macrophages, neutrophils and monocytes. When each of these factors are not at their strongest at the same time, they are together less likely to produce the catastrophic sepsis that needs all of them.
Another clock function is to limit the duration of the responses. For example, only have BMAL1 operate at one particular peak time. Different factors are regulated at different times that will limit the length and strength of one of the factors. The goal is to avoid an all-out response that will cause severe danger.
 Another mechanism is limiting travel of cells to the sites at different times. For example, neutrophils and monocytes are limited by different cycles. When they aren’t limited, there is more tissue damage and sepsis.
Another mechanism is limiting travel of cells to the sites at different times. For example, neutrophils and monocytes are limited by different cycles. When they aren’t limited, there is more tissue damage and sepsis.
During particular severe infections, these limiting gating cycles are disrupted to increase the amount of needed inflammation. New gene networks are stimulated during a severe infection that overrides these clock related events. These increase tissue damage and the danger of catastrophe.
Immune responses
 Every part of the immune response operates on 24 hour cycles. The number of cells produced, the risk of getting a new infection and septic shock, and production of cytokines are all in cycles. These cycles are affected by signals from outside of the cell and internal processes.
Every part of the immune response operates on 24 hour cycles. The number of cells produced, the risk of getting a new infection and septic shock, and production of cytokines are all in cycles. These cycles are affected by signals from outside of the cell and internal processes.
The production and release of stem cells to make immune blood cells throughout the body is stimulated by rhythmic signals from the sympathetic nervous system. Norepinephrine stimulates production of cytokine CXCL12 in the bone marrow. When the circadian rhythm produces less CXCL12 then more immune cells travel from the bone marrow into the blood and tissues.
Steroid secretion (glucocorticoids in the adrenal) cycles affect the amount of T and B cells in the blood with opposite peaks and nadirs. T cells themselves have cycles that influence other cells based on the time of day. These T cell cycles are influenced by dendritic and macrophage cells that present antigens.
 In fact, the adaptive immune response, which responds slowly appears to be more dependent on factors outside of the cell than the internal individual cellular clocks.
In fact, the adaptive immune response, which responds slowly appears to be more dependent on factors outside of the cell than the internal individual cellular clocks.
In innate cells that respond rapidly, intrinsic factors appear to be more important. Internal 24 hour clocks inside of individual cells appear to be very important for monocyte and macrophage behavior. But, even though these inherent core rhythms are active, they can be over rode by signals from outside. LPS from microbes (lipopolysaccharides on bacteria surfaces) alters the cycle length and strength. This shifts the cycles to control from the outside. Larger anti-inflammatory effects of the steroids can also be altered when particular inflammation occurs.
Clocks Against Inflammation
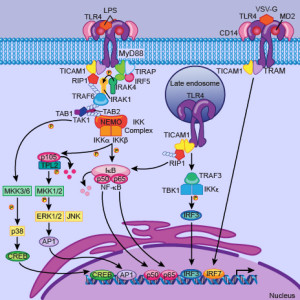 The cycles of innate immune cells are able to respond to inflammation by working against it as well as stimulating it. The genetic cycles in the immune cells that are directly connected with production of cytokines have a set of unique protein transcription factors that can alter the oscillations. The ordinary CLOCK/BMAL1 factors are coordinated with triggering of cytokine genes and can increase or slow production related to daily cycles. CRY proteins are part of this regulatory process both increasing and decreasing inflammation cytokines. Some of the complex cycles described in the post on metabolism and circadian rhythms are part of this process.
The cycles of innate immune cells are able to respond to inflammation by working against it as well as stimulating it. The genetic cycles in the immune cells that are directly connected with production of cytokines have a set of unique protein transcription factors that can alter the oscillations. The ordinary CLOCK/BMAL1 factors are coordinated with triggering of cytokine genes and can increase or slow production related to daily cycles. CRY proteins are part of this regulatory process both increasing and decreasing inflammation cytokines. Some of the complex cycles described in the post on metabolism and circadian rhythms are part of this process.
There are many ways this plays out. One is related to the transcription factor NF-kB that increases inflammation. When LPS overrides the mechanisms, NF-kB is involved in several ways. One way is by interring with the transcription factor CLOCK. This works because CLOCK directly increases its activity by stimulation a particular region of the molecule. Inflammatory genes are decreases in a particular rhythm. Interfering with BMAL1 does the opposite.
 Another mechanism involves the factors REV-ERB, which is the negative feedback loop, is involved in affecting thousands of genes in immune cells. In this case, they stop a particular set of genes that produce inflammation for a particular part of a cycle.
Another mechanism involves the factors REV-ERB, which is the negative feedback loop, is involved in affecting thousands of genes in immune cells. In this case, they stop a particular set of genes that produce inflammation for a particular part of a cycle.
A third way involves the receptor for glucocorticoid steroid secretion. This normally synchronizes many cellular clocks. But, they don’t affect the inherent clock in macrophages. In this mechanism, the cell blocks the effects of the glucocorticoids.
How Does This All Work
 While it is clear that all immune functions are regulated by clocks, how they interrelate is not clear. The genetic and epigenetic mechanisms regulating individual cell clocks that are being discovered are very complex. Much also depends on the interactions of external factors related to the core clock genes and the genes particular to each type of cell. Some is directed from the most core master clock proteins CLOCK and BMAL1. Some are related to downstream loops such as REV-ERB and CRY.
While it is clear that all immune functions are regulated by clocks, how they interrelate is not clear. The genetic and epigenetic mechanisms regulating individual cell clocks that are being discovered are very complex. Much also depends on the interactions of external factors related to the core clock genes and the genes particular to each type of cell. Some is directed from the most core master clock proteins CLOCK and BMAL1. Some are related to downstream loops such as REV-ERB and CRY.
How circadian clocks influence metabolic cycles is not clear, but many of the very complex loops are being found. How these immune cycles and metabolic cycles interact is being worked on but is vastly complex involving central clocks, many kinds of individual cellular clocks, and a large number of genetic regulatory layers. The extent of the influence of outside signals on innate immunity is not clear. There are definite important cycles in cyanobacteria where the photosynthesis during sunlight and nitrogen fixation at night. But, it is not clear if such cycles exist in immune cells related to the types of cells triggered.
Individual Cell Clocks and Immunity
 Research shows that all aspects of life and physiology are based on communication among cells. Cells know where they should be, where they should go, how big they should be, and where to stop at the edge of an organ. Capillary cells signal to determine actions of the tissues, but also among immune cells they call for help in particular ways and from particular regions. Microbes join in the signaling with lining cells in the gut and elsewhere to determine digestion and how dangerous they will become.
Research shows that all aspects of life and physiology are based on communication among cells. Cells know where they should be, where they should go, how big they should be, and where to stop at the edge of an organ. Capillary cells signal to determine actions of the tissues, but also among immune cells they call for help in particular ways and from particular regions. Microbes join in the signaling with lining cells in the gut and elsewhere to determine digestion and how dangerous they will become.
Cellular communications has been shown to be the basis of all physiology and life, itself. New research is uncovering a vast area of signaling among all cells related to overlapping and interacting clocks.