 Most people are familiar with proteins regulating genes and peptides and amino acids as signals and factors in the brain. Now, fat molecules are found to be vital signals for a wide range of brain functions, including protecting neurons from cell death, stimulating synapses and creating new neurons. In particular, polyunsaturated fatty acid signaling in the brain is critical for cognition, pain and mood. Because proteins are made directly from DNA and messenger RNA, protein effects can be studied by “knocking out” the specific gene for a particular protein. Studying fatty acids is far more complex, since there is no simple way to observe them and they are rapidly metabolized in vast, complex pathways. Research is just now uncovering their astonishing variety and significance.
Most people are familiar with proteins regulating genes and peptides and amino acids as signals and factors in the brain. Now, fat molecules are found to be vital signals for a wide range of brain functions, including protecting neurons from cell death, stimulating synapses and creating new neurons. In particular, polyunsaturated fatty acid signaling in the brain is critical for cognition, pain and mood. Because proteins are made directly from DNA and messenger RNA, protein effects can be studied by “knocking out” the specific gene for a particular protein. Studying fatty acids is far more complex, since there is no simple way to observe them and they are rapidly metabolized in vast, complex pathways. Research is just now uncovering their astonishing variety and significance.
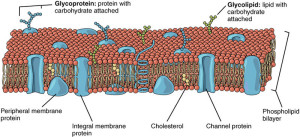 A previous post described the great complexity of cell membranes, which are made from phospholipids. Fatty acids attach to phospholipids in the cell membrane, including neurons and glia. When needed, fatty acids are cut from their membrane attachments to be altered into derivatives and mediators that participate in a wide range of signaling cascades.
A previous post described the great complexity of cell membranes, which are made from phospholipids. Fatty acids attach to phospholipids in the cell membrane, including neurons and glia. When needed, fatty acids are cut from their membrane attachments to be altered into derivatives and mediators that participate in a wide range of signaling cascades.
Derivatives and mediators metabolized from fatty acids have important functions in the survival of the neuron, the firing of the action potential, the production of new neurons, the maintenance of synapses, and regulation of inflammation in the brain, including neuro-inflammation throughout the body (see post on Inflammation Pathways in Neuroplasticity). They are, also, important regulators of mood and cognitive processes, integrally related to many brain diseases, such as Alzheimer’s and depression (see post Inflammation and Dementia).
While the enormous complexity of their actions are just being uncovered, this post will discuss what is known about fatty acids and their brain functions.
Fats are One Type of Lipid
 Lipids in the neuron are described in the post Amazing Complexity of the Membrane. They either don’t like water (hydrophobic—such as oil in salad dressing, where the oil and vinegar separate) or they do like water, hydrophilic. Many have one end that doesn’t mix with water and the other end can form loose bonds with water. The cell membrane is made of two layers of lipids. Other lipids form membranes for vesicles and intracellular organelles.
Lipids in the neuron are described in the post Amazing Complexity of the Membrane. They either don’t like water (hydrophobic—such as oil in salad dressing, where the oil and vinegar separate) or they do like water, hydrophilic. Many have one end that doesn’t mix with water and the other end can form loose bonds with water. The cell membrane is made of two layers of lipids. Other lipids form membranes for vesicles and intracellular organelles.
There are a large number of different kinds of lipids, including fats (fatty acids), waxes, sterols (cholesterol) and vitamins (K, E, D, A). Glycerides (triglycerides, diglycerides and monoglycerides) and phospholipids are made from fatty acids. The 8 categories of lipids are determined by two basic building blocks—acids and isoprene
Fatty Acids in Food and Physiology
 Fatty acids are made from condensed ketoacyl groups. In ordinary speech, “fat” and “fatty acid” are used interchangeably. Scientifically, fatty acids are a type of fat. Fat in foods consists mostly of fatty acids bound to a glycerol (an alcohol) molecule by an “ester” bond (discussed later). Triglycerides are the most common fat in food and the most used and stored in the body. After entering the body and being metabolized, they becomes mono and di glycerides.
Fatty acids are made from condensed ketoacyl groups. In ordinary speech, “fat” and “fatty acid” are used interchangeably. Scientifically, fatty acids are a type of fat. Fat in foods consists mostly of fatty acids bound to a glycerol (an alcohol) molecule by an “ester” bond (discussed later). Triglycerides are the most common fat in food and the most used and stored in the body. After entering the body and being metabolized, they becomes mono and di glycerides.
Fatty acids attach to phospholipids in the cell membranes, where they await being detached and utilized. The ester bonds holding triglycerides together are broken and free fatty acids are released.
Ingested as triglycerides, fatty acids are broken down by pancreatic enzymes to be absorbed by the intestinal cell. For this process, they need to be emulsified by bile acids for the enzymes to work. Emusification is a process of mixing together two materials (like oil and vinegar in salad dressing) that normally won’t stay mixed
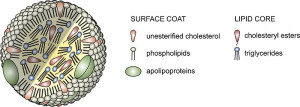 When fatty acids are absorbed from the intestine, special lipoproteins or chylomicron transport them to the liver and other fat tissues. (Because they dislike water, they cannot travel alone.) Then they bind to the membranes of liver, fat and muscle cells. Then, they are transported to the brain.
When fatty acids are absorbed from the intestine, special lipoproteins or chylomicron transport them to the liver and other fat tissues. (Because they dislike water, they cannot travel alone.) Then they bind to the membranes of liver, fat and muscle cells. Then, they are transported to the brain.
What is a Fatty Acid?
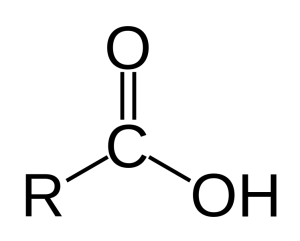 In chemistry, a fatty acid is a long chain of carbon molecules (R in the diagram to the left) with two oxygen molecules attached at one end. The end with the two oxygen is called an “acid.” One of the oxygen molecules has a double bond with a carbon. The other oxygen has a hydrogen (noted as OH). The OH can react with water and other ionic molecules.
In chemistry, a fatty acid is a long chain of carbon molecules (R in the diagram to the left) with two oxygen molecules attached at one end. The end with the two oxygen is called an “acid.” One of the oxygen molecules has a double bond with a carbon. The other oxygen has a hydrogen (noted as OH). The OH can react with water and other ionic molecules.
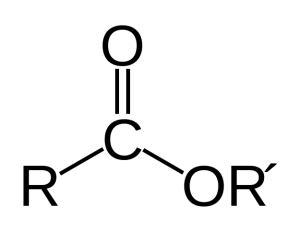
A very significant reaction is when a fatty acid attaches to other molecules, such as the phospholipids and lipoproteins that are sticking out of the cell’s membrane. This attachment is called an “ester” or “esterification” and occurs when the hydrogen in the OH is replaced with an attachment to another molecule; therefore the oxygen becomes a bridge between the fatty acid and the other molecule. (R’ is the lipoprotein in the diagram on the right). In this way many fatty acids are hanging from molecules in the membrane and are ready to be cut off and used for many different purposes.
Fatty acids are described by length and by the number of double bonds. Long chain fatty acids have more than 12 carbons in a row, 22 or more are very long chain.
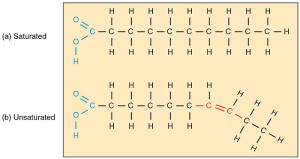
Saturated fatty acids have no double bonds between carbons; that is, every carbon has all four bonds used up either connecting with other carbons and 2 hydrogen. In the brain, palmitic acid and stearic acid are saturated fatty acids.
Unsaturated means there are double bonds between some of the carbons. There are mono unsaturated fats with one double bond, and poly with multiple double bonds between different carbons. Oleic is the main mono-unsaturated (only one double bond). Poly unsaturated have many double bonds.
The beginning of the fatty acid is the acid group (O=C-OH), and the end is the last carbon. Omega refers to the end of the fatty acid molecule. Omega-3 refers to the third carbon from the end. If that third carbon from the end has a double bond, the fatty acid is called an Omega-3 unsaturated fatty acid.
![]() Omega-6 unsaturated fatty acids have a double bond using the sixth carbon from the end of the molecule.
Omega-6 unsaturated fatty acids have a double bond using the sixth carbon from the end of the molecule.
Poly unsaturated omega-3 and omega-6 have more than one double bond starting with either the third or the sixth carbon in the molecule. There can be three, five or six double bonds—ALA has three; EPA has five; and DHA have six double bonds. There are many of each type, but only a small number in the brain.
Shape Determines Signaling
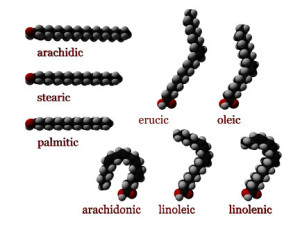
As with proteins, the shape of the fat molecule is the all-important factor determining its signaling functions. Shape is determined by whether the hydrogen atoms are on the same sides of the double bond—called cis—versus on the opposite—called trans. Cis fatty acids have more bending with unique curved shapes. Trans unsaturated fatty acids are flat, similar in shape to saturated fatty acids and they both cause some of the same health problems.
With cis unsaturated fatty acids, the more double bonds there are, the more rigid is the shape and therefore the more particular is the signal. With many cis bonds and many double bonds, the molecule has dramatic rigid curves.
Fatty Acids in Food

Fatty acids in nature have an even number of carbons—4 to 28. In food, we get them from triglycerides or phospholipids. They provide good energy producing a large amount of ATP. Heart and skeletal muscles prefer fatty acids to glucose for energy. Recently, some animal studies show brain cells using fatty acids for fuel, as well—breaking the dogma that brain cells only use sugar.
Omega-3 unsaturated fatty acids in human physiology are ALA from plants (nuts, algae, flax, hemp, sage, etc.), EPA and DHA often in oils from fish, algae and plankton from the ocean. Taking supplements of omega-3s possibly help many diseases including cancer and heart disease.

Omega-6 unsaturated fatty acids come from arachidonic acid in the cell membrane and have complex effects, some negative. They are found in a wide range of processed cooking oils. They produce prostaglandin and eicosanoids during inflammation, which are the targets of medications (NSAIDs). Omega-6 products can stimulate asthma, arthritis, clots and heart disease. They promote inflammation and cancer. They compete with omega-3s for storage space and participation in metabolism.
Brain Fatty Acids
 The two prominent poly unsaturated fatty acids in the brain are ARA (arachidonic acid) an omega-6 fatty acid, and DHA (docosahexaenoic acid) an omega-3. Others, such as LNA, EPA and ALA are either not present or only in very tiny amounts.
The two prominent poly unsaturated fatty acids in the brain are ARA (arachidonic acid) an omega-6 fatty acid, and DHA (docosahexaenoic acid) an omega-3. Others, such as LNA, EPA and ALA are either not present or only in very tiny amounts.
In the brain, fatty acids are attached to phospholipids in the plasma membrane. DHA is found on phospholipids in synapses and neuronal cell bodies. Palmitate and oleate are attached in myelin.
When activated by neurotransmitters, DHA and ARA are released from the membrane and are used as brain signals. In fact, they are altered into many derivatives that are even more powerful signals. DHA and ARA are attached 10,000 times greater than free. There is an elaborate regulation of the amount that is cut off and utilized for signaling.
Fatty Acid Transporters in the Brain
 There is still a lot of controversy about how and when free fatty acids enter the brain and how they enter brain cells. Free fatty acids can diffuse through membranes without proteins transporters, but it is not clear if this happens in the brain.
There is still a lot of controversy about how and when free fatty acids enter the brain and how they enter brain cells. Free fatty acids can diffuse through membranes without proteins transporters, but it is not clear if this happens in the brain.
On the other hand, transporters are very complex and appear to trap them and take them to different pools and compartments for storage. They might enter with phospholipids and lipoproteins. There are special receptors and transporters for attached DHA that have been identified. It is not clear how much supplements help these processes.
 It has been thought that the brain is not able to manufacture DHA and ARA, which are supplied by blood transport from food. In fact, the brain has enzymes to make ARA and DHA, but doesn’t seem to use them.
It has been thought that the brain is not able to manufacture DHA and ARA, which are supplied by blood transport from food. In fact, the brain has enzymes to make ARA and DHA, but doesn’t seem to use them.
Most of the supply comes from the liver. The brain can make saturated fatty acids and those fatty acids with one unsaturated site, but doesn’t make poly unsaturated DHA and ARA. The polyunsaturated linoleic acid (LNA) and α-linoleic acid (ALA) come from food and can be metabolized into ARA and DHA in the liver. There are, also, stores of DHA and ARA kept in fat tissues that are released as needed and transported on albumin to the brain.
Upon entering the brain, ARA and DHA are esterified to phospholipids or oxidized. There are a number of different metabolic compartments for them, but this is not well understood. There are many specific pathways for the particular creation of derivatives for each.
ARA, DHA Cut from Membranes When Activated by Neurotransmitter Receptors
 When neurotransmitter receptors are stimulated (dopamine, cholinergic, serotonin, NMDA), enzymes are stimulated that cut ARA and DHA free from attachment to lipoproteins in the membrane. ARA and DHA, then, enter the complex metabolic cycles to make derivatives and mediators.
When neurotransmitter receptors are stimulated (dopamine, cholinergic, serotonin, NMDA), enzymes are stimulated that cut ARA and DHA free from attachment to lipoproteins in the membrane. ARA and DHA, then, enter the complex metabolic cycles to make derivatives and mediators.
ARA responds to activation of dopaminergic, cholinergic, serotonergic or glutamate NMDA receptors. DHA responds to cholinergic or serotonergic receptor activation. Two different enzymes cut the attachment of ARA and DHA from phospholipids in the neuronal membrane. This detachment is carefully regulated.
After release, ARA and DHA are attached to other phospholipids through they are not well understood. They participate in the very complex Lands cycle, which manufactures a wide range of derivatives and mediators. This process uses 5% of the brain’s energy.
During ischemia and inflammation, receptors, also, release DHA and it enters the Land’s cycle to make helpful molecules.
Mediators from DHA and ARA
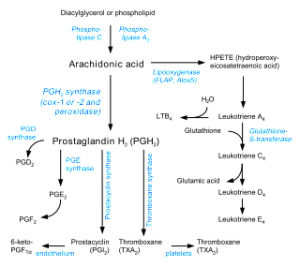
Released from the membrane, DHA and ARA are altered into active signals, called mediators, critical for regulation and responses of the immune response including clearance of debris. One well-known enzyme creating mediators is COX – (cyclooxygenases). There are many others, such as LOX and P450.
Many different derivatives of ARA have been discovered in the brain and a smaller number for DHA. Many ARA mediators produce inflammation, calling for leukocytes and cleaning of debris. In neurons COX2 changes ARA to prostaglandin, a very strong signal in the neuron. COX2 inhibitors are the well-known NSAID medications for inflammation and pain.
Glial cells, also, make many derivatives and are active in the entire process.
Derivatives of DHA and ARA are important signals in the brain. They help regulate the hypothalamus to produce glucose from the liver and regulate the effects of food intake. They are critical in pain.
Endocannabinoids Are Critical Derivatives of ARA and DHA

DHA and ARA metabolites regulate the very dynamic changes in the cell membrane (see post on the Amazing Complexity of the Cellular Membrane). Derivative molecules activate receptors stimulating signaling cascades. Some free fatty acids stimulate a wide range of different receptors including very important protein kinase C (PKC) and inhibition of the nuclear factor, NF-κβ.
Endocannabinoids are neurotransmitters made from polyunsaturated fatty acids that are attached to phospholipids in the cell membrane. They travel from the postsynaptic neuron to the pre synaptic in retrograde fashion and regulate many processes in the synapse. More are made from ARA than DHA.
Cannabinoids are produced by neurons, astrocytes and microglia. The fatty acids anandamide ethanolamide (AEA) and 2-AG (2-arachidonogycerol) are the two most common endocannaboids in the brain. These bind to the CB1 and CB2 receptors.
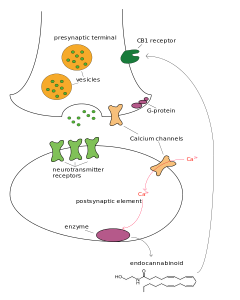 Endocannabinoids regulate release of glutamate, GABA, monoamines, opiods and acetylcholine. They are secreted backwards from the postsynaptic to the pre synaptic CB receptors, as retrograde neurotransmitters and are critical in neuroplasticity for excitatory and inhibitory synapses.
Endocannabinoids regulate release of glutamate, GABA, monoamines, opiods and acetylcholine. They are secreted backwards from the postsynaptic to the pre synaptic CB receptors, as retrograde neurotransmitters and are critical in neuroplasticity for excitatory and inhibitory synapses.
With more ARA and DHA in high fat diets, there is a greater amount of endocannabinoids. They have many other interactions with other fatty acids, such as EPA. Endocannabinoids functions are just being discovered, but it is known that they regulate signaling in prefrontal cortex and nucleus accumbens.
DHA and SNARE
 The Soluble NSF Attachment protein Receptor is known as SNARE and is a large protein family (more than 60 types) that provide the ability of vesicles and cell membranes to fuse.
The Soluble NSF Attachment protein Receptor is known as SNARE and is a large protein family (more than 60 types) that provide the ability of vesicles and cell membranes to fuse.
DHA is critical for the SNARE mechanism whereby the neurotransmitter vesicles are merged with the neuron membrane. They affect assembly of the SNARE complex with many complex interactions. They are critical for the dendrite structures in the spine and the postsynaptic density 95. They are needed for NMDA receptors
Neurogenesis and Neuroprotection
 How DHA helps memory and learning is not clear. It is known to increase survival of neurons and to stimulate the creation of new neurons in the learning process. DHA is the major constituent of phosphatidylserine, which produces factors that are necessary for neurogenesis.
How DHA helps memory and learning is not clear. It is known to increase survival of neurons and to stimulate the creation of new neurons in the learning process. DHA is the major constituent of phosphatidylserine, which produces factors that are necessary for neurogenesis.
One major metabolite of DHA is synaptamide, which stimulates new dendrites and synapses. Synaptamide is more powerful than DHA itself. Giving animals synaptamide stimulates stem cells for new neurons. Other factors stimulate a pathway that stops neuronal cell death. The level of DHA is correlated with maintaining the critical factor BDNF that is necessary to maintain neurons and make new ones.
Inflammation—DHA and ARA
 Mediators of DHA dramatically decrease inflammation outside of the brain. They possibly can decrease brain disorders that are influenced by inflammation, (see post Inflammation and Dementia) perhaps Alzheimer’s, Parkinson’s and depression. They help diseases affected by inflammation.
Mediators of DHA dramatically decrease inflammation outside of the brain. They possibly can decrease brain disorders that are influenced by inflammation, (see post Inflammation and Dementia) perhaps Alzheimer’s, Parkinson’s and depression. They help diseases affected by inflammation.
Giving both EPA and DHA lowers inflammation cytokines and increases astrocytes. DHA appears to directly stop macrophages and microglia in the brain.
Molecules derived from ARA start inflammation, but ARA is, also, critical in stopping inflammation. COX enzymes produce prostaglandins from ARA, which increases inflammation. But, prostaglandins can, also, trigger LOX derivatives of DHA to stop inflammation.
DHA and the mediator neuroprotectin alter the way amyloid is created. It decreases the effect of beta secretase BACE1, as well as activating alpha secretase, making the amyoid factors less likely to become Alzheimer’s.
It is difficult to separate several effects of DHA, such as protecting neurons and stopping inflammation. The effects of ARA are difficult to separate from its derivatives.
DHA and ARA Compete for Places in Metabolism Pathways
 Low DHA and ARA levels occur through increased metabolism or decreased intake. In all studies, the causes are not clear between these two. It is not really possible to measure the brain effects of DHA from diet through current technology.
Low DHA and ARA levels occur through increased metabolism or decreased intake. In all studies, the causes are not clear between these two. It is not really possible to measure the brain effects of DHA from diet through current technology.
But, it is clear that ARA and DHA replace each other. They are competitive for the metabolic cycles. There are different pools of DHA in the different brain regions such as thalamus, hippocampus and occipital cortex. It takes three months of diet lack to lower brain levels, but the daily competition is not well understood.
It is possible that low DHA is part of early Alzheimer’s. There is a similar question about depression and bipolar disorder. Oxidative stress reduces DHA and RHA by using them up in metabolism.
DHA and Mood
There is a lot of interesting, but unclear, data related to DHA and mood. Studies have not been consistent in the treatment of depression.

More DHA in the blood appears to correlate with lower anxiety and depression and ARA the opposite. There are no differences in ARA and DHA amounts in the frontal cortex with depression or bipolar. It is possible that lithium affects fatty acid metabolism. Decreased diet of DHA is correlated with prevalence of depression. There are some benefits in depression studies, but they are not consistent. Perhaps it helps severe depression, but not mild. It is possible that mostly EPA is better, but it is not certain. COX2 inhibitor pain medications may help bipolar disorder.
In animals, deprivation of DHA causes depression and anxiety and lowers DHA brain levels in PFC and hippocampus. Long-term deprivation impairs monoamines. Increases in diet increased serotonin, dopamine and DHA. DHA and ARA have complex relations with cortisol, a marker for depression. Normal people have less anxiety with DHA and less cortisol effect with stress. A low diet of DHA exaggerated the stress response; supplements prevented this.
DHA may have many different effects on depression in different circumstances. It may help during a brain hypothalamic-adrenal stress reaction and neuroinflammation. Currently, there is not enough information to use them in treatment.
Cognition
The effects of DHA in cognition are, also, inconsistent.

DHA in the brain increases until age 20. Animal studies show a critical period in childhood when they are necessary for cognition. Low levels of DHA are associated with ADHD, poor memory and autism. As with all these studies, it is not clear which comes first—low diet or more metabolism of the DHA. It is, also, very complex because the metabolism is so diverse and poorly understood.
If cytokine interleukin1β is given experimentally, it causes memory deficits and anxiety; EPA helps improve this memory. It, also, helps negative affects of microglia.
DHA possibly helps increase cognition. In human studies, supplementation has not shown increased cognition or memory in humans. They did help animals in stress and aging, but not in normal conditions.
DHA has not helped dementia patients. It is possible there is a unique effect in patients with APOE4 carriers, one group of dementia patients with a hereditary link (see post Inflammation and Dementia). In animals, with more DHA there are less amyloid plaques. There is a lot of conflicting research in animals in this field with many different variables that are not well understood.
Schizophrenia
In patients with schizophrenia, there is reduced DHA in plasma and RBC in those treated and untreated. Brain studies after death showed 20% lower DHA in brains, but not in frontal lobes. ARA is decreased in schizophrenia brains probably from oxidative stress. Giving DHA might help. Zyprexa decreases ARA turnover, COX2 expression and prostaglandin; mood stabilizers decrease ARA
Complexity in Effects of Supplement Use
Because of the great complexity of the uptake of DHA and ARA in the brain and neurons, it is not clear exactly what supplements do and in what circumstances they help.
 In healthy people, DHA brain uptake is 4 mg per day and ARA is 18 mg per day. There are 5 grams of DHA in the brain. If decreased by a third, it would take a year to bring it back to the normal level. It is not clear if supplements increase brain uptake rate.
In healthy people, DHA brain uptake is 4 mg per day and ARA is 18 mg per day. There are 5 grams of DHA in the brain. If decreased by a third, it would take a year to bring it back to the normal level. It is not clear if supplements increase brain uptake rate.
In seizures, omegas reduced seizures after three months of oral treatment. Free DHA given subcutaneously helped immediately. In stroke models, DHA helped protect future damage. And free DHA given intravenously helped spinal cord injury. So, the way it is given may be very important.
It is, also, not clear if it is DHA or its derivatives that help. It is not clear if more dietary DHA increases meditators. A trial of a CB1 antagonist caused psychiatric problems and the study was stopped. The complexity is just too great at the current time. Altering fatty acids can have wide ranging effects on mood, pain and cognition, but it is not clear exactly what they are.
Polyunsaturated Fatty Acid Signaling in the Brain
 An enormous new complexity has been found in brain signaling related to fatty acids. The two prominent polyunsaturated fatty acids, DHA and ARA, enter the brain from the blood after either being eaten or synthesized in the liver. They are, also, stored in fat deposits for later use.
An enormous new complexity has been found in brain signaling related to fatty acids. The two prominent polyunsaturated fatty acids, DHA and ARA, enter the brain from the blood after either being eaten or synthesized in the liver. They are, also, stored in fat deposits for later use.
They attach to phospholipids in the neuron and glia membranes and remain as an enormous reservoir of molecules that at any time can be detached and metabolized into many powerful signals. The metabolic pathways that make these derivatives and mediators are extremely complex and science has just scratched the surface of their vast effects.
The many important powerful fatty acid signals are another layer of added complexity in brain signaling. As well as the thousands of types of neurons, the vast neuronal network, electrical synapses and gradients, the very complex astrocyte network and extremely active complex brain waves, yet another vast signaling network has been found with polyunsaturated fatty acids and their derivatives.