 Cancers demonstrate that communication among a variety of wide cell types is vital to their survival. Cancers are able to convert many local supportive cells to become comrades in initiation, support, growth, and metastasis of the cancer. It is remarkable how they can trick immune cells, connective tissue and mesenchymal cells, epithelial and blood vessel cells, and neurons to become supportive.
Cancers demonstrate that communication among a variety of wide cell types is vital to their survival. Cancers are able to convert many local supportive cells to become comrades in initiation, support, growth, and metastasis of the cancer. It is remarkable how they can trick immune cells, connective tissue and mesenchymal cells, epithelial and blood vessel cells, and neurons to become supportive.
They also attract help from brain cells. Among all organs, the brain has a unique immune environment that has been noted in multiple previous posts. This is somewhat based on the unique anatomical structures that keep the brain tissue separate (see post).  Another previous post showed how neurons, both in the brain and outside of it, can be vital for the development of cancer. This post describes how many kinds of brain cells, even within a very unique environment, converse with cancers and help them in many different ways. Supportive brain cells help cancers via communication.
Another previous post showed how neurons, both in the brain and outside of it, can be vital for the development of cancer. This post describes how many kinds of brain cells, even within a very unique environment, converse with cancers and help them in many different ways. Supportive brain cells help cancers via communication.
Since the brain has unique immune responses, multiple barriers to entry, and distinctive glial cells, the process in the brain is different from any other part of the body. The brain also has a unique type of matrix in between cells. Recently, lymphatic drainage has also been described in meninges and the lymph nodes of the neck. Some cancers alter the barriers of the blood, CSF, and brain allowing many other cells in. This post focusses on one of the worst, most aggressive cancers (glioblastoma, meduloblastomas and metastases from other cancers, the most common being lung cancers and melanoma). Each type uses different conversations with the local, supportive cells.
Microglia and Other Macrophages Help Cancers
 Microglia are a type of macrophage that migrated to the brain in the fetus and then stayed there for life. They live long and reproduce in the brain. When they need reinforcements, microglia call for other macrophages that live near the blood vessels to enter the brain and help fight infections. While starting as immune cells and being the primary brain immune cells, microglia also are highly involved in protecting a small region of the brain territory that it surveils each day by touching everything. Microglia are very involved in supporting the neuronal and astrocyte networks and help to eat (prune) unnecessary synapses.
Microglia are a type of macrophage that migrated to the brain in the fetus and then stayed there for life. They live long and reproduce in the brain. When they need reinforcements, microglia call for other macrophages that live near the blood vessels to enter the brain and help fight infections. While starting as immune cells and being the primary brain immune cells, microglia also are highly involved in protecting a small region of the brain territory that it surveils each day by touching everything. Microglia are very involved in supporting the neuronal and astrocyte networks and help to eat (prune) unnecessary synapses.
While in general macrophages often are in all cancers, they are the most common immune cell in brain cancers. They can be a third of the mass of a tumor, including both microglia and macrophages from the bone marrow. Monocytes from the blood also enter the brain and cancers, called by signals and then multiplying in the local tissue. These cells are often able to enter because of the alterations in the blood brain barrier cells that allow more travel into the brain and more inflammation. It is hard for research to distinguish these sources of microglia versus other macrophages, (often using the amount of the receptor CD45 as a marker, which is low in microglia and high in macrophages; but this only works in mice, not humans). A new human marker is Tmem119 and it is in microglia only. There are other markers, but more recent research shows that migrating macrophages can adjust and develop the same markers as the microglia. These gene networks are stimulated by the cancer micro environment signals. A new marker was also recently found for macrophages (CD49D).
 More complexity has been found in macrophages than a simple shift from M1 to M2 types. In brain cancers, macrophages are more often helpful to the cancer. They don’t call for T cells much and don’t even have the receptors to help activate T cells (CD40, CD80, CD86). Generally, the more macrophages in the tumor, the worse the cancer. New drugs aim to block these macrophages.
More complexity has been found in macrophages than a simple shift from M1 to M2 types. In brain cancers, macrophages are more often helpful to the cancer. They don’t call for T cells much and don’t even have the receptors to help activate T cells (CD40, CD80, CD86). Generally, the more macrophages in the tumor, the worse the cancer. New drugs aim to block these macrophages.
Macrophages that are activated have many signals that regulate the activity of cancer stem cells. They stimulate new blood vessels and seem to block treatments to stop new blood vessels. Their signals seem to help metastatic behavior. Some treatments have been able to change macrophage behavior.
Dendritic Cells Help Cancers in the Brain
 Dendritic cells (DCs) are produced in bone marrow in the myeloid line and are vital to present antigens to stimulate T cell activity fighting cancer and microbes. Microglia present most antigens in the brain to T cells. But, there is recent interest in stimulating DCs to activate T cells along with checkpoint inhibitors. Also, vaccines that stimulate DCs appear to help also. One technique is giving a different stimulus than the cancer to activate DCs such as toxins (tetanus). One particular cytokine (CCL3), a chemokine that calls to a location, helped. Other therapies stimulate the cancer to produce other antigens that stimulate attack.
Dendritic cells (DCs) are produced in bone marrow in the myeloid line and are vital to present antigens to stimulate T cell activity fighting cancer and microbes. Microglia present most antigens in the brain to T cells. But, there is recent interest in stimulating DCs to activate T cells along with checkpoint inhibitors. Also, vaccines that stimulate DCs appear to help also. One technique is giving a different stimulus than the cancer to activate DCs such as toxins (tetanus). One particular cytokine (CCL3), a chemokine that calls to a location, helped. Other therapies stimulate the cancer to produce other antigens that stimulate attack.
Neutrophils Help Cancers in the Brain
 As the most common white blood granulocyte ( a cell that has granules or lobes by microscope observation), most studies have been on leukocytes. Studies are consistent with stimulation of gliomas using the special protein S100. These proteins seem to stimulate metastasis. They appear as leukocytes are being called to the brain and then metastasis occurs. This is similar to other organs. It appears that as the unique immune restrictions in the brain are broken down, inflammation helps cancer progression. Most leukocytes compared with lymph cells is a bad sign.
As the most common white blood granulocyte ( a cell that has granules or lobes by microscope observation), most studies have been on leukocytes. Studies are consistent with stimulation of gliomas using the special protein S100. These proteins seem to stimulate metastasis. They appear as leukocytes are being called to the brain and then metastasis occurs. This is similar to other organs. It appears that as the unique immune restrictions in the brain are broken down, inflammation helps cancer progression. Most leukocytes compared with lymph cells is a bad sign.
Even Lymph Cells Help Cancers in the Brain
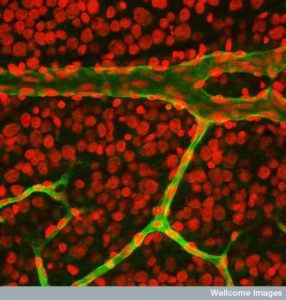
 Lymph vessels take fluid, waste, and immune cells from the CSF and blood vessels. Lymphatics have been found in the meninges recently (see post). It is not yet clear if there is direct drainage from the brain tissues, but there appears to be (glymphatics). It is not yet clear how cancer interacts with these vessels.
Lymph vessels take fluid, waste, and immune cells from the CSF and blood vessels. Lymphatics have been found in the meninges recently (see post). It is not yet clear if there is direct drainage from the brain tissues, but there appears to be (glymphatics). It is not yet clear how cancer interacts with these vessels.
In the unique brain environment, there are sometimes unique T cell responses. One treatment where CD8+ cells were infused altered T cells in new ways. Even without the same inflammation that occurs in other organs, the local environment in the brain alters T cells. By inhibiting checkpoint pathways in the T cells, they can be made to be more aggressive attacking the cancer. Checkpoints are the ways that T cells are able to modify their aggressiveness and tamp down inflammation. By inhibiting these pathways, they become more active and aggressive toward cancers.
Another technique is to stop the types of regulatory T cells that tamp down inflammation normally, such as avoiding food allergies. With antibodies the effects of these cells can be decreased (decrease of CD25).
Barrier Cells Are Influenced to Help Cancers
 Nighty eight percent of all molecules are kept out of the blood brain barrier (BBB). Cancers can sponsor leakiness. This allows some of the antibody treatments in. As well as pericytes surrounding brain blood vessels and astrocytes for protection, microglia repair BBB after injury. Cancer signals also influence the function of the BBB. Alterations in the well-known and influential Wnt pathway for example has subtypes that determine how good the BBB will work by allowing in more medications. A sonic hedgehog (SSH) type signal keeps BBB strong and this cannot be treated with medications yet.
Nighty eight percent of all molecules are kept out of the blood brain barrier (BBB). Cancers can sponsor leakiness. This allows some of the antibody treatments in. As well as pericytes surrounding brain blood vessels and astrocytes for protection, microglia repair BBB after injury. Cancer signals also influence the function of the BBB. Alterations in the well-known and influential Wnt pathway for example has subtypes that determine how good the BBB will work by allowing in more medications. A sonic hedgehog (SSH) type signal keeps BBB strong and this cannot be treated with medications yet.
Metastatic cells can find the brain a good place to live if they can get in. They are protected from a lot of immune responses and medications. They sometimes use enzymes to break the tight junctures of the barrier cells. They produce signals copied from immune cells to gain permission to enter. Multiple unique signals have been identified that they use for entry.
New Capillaries in Brain Help Cancers
 The new blood vessels grown by the cancer are different than ordinary brain vessels and are leaky. Leakiness in the tumor causes low oxygen, tissue damage, and fluid buildup (edema). Some therapies have attacked these new blood vessels with limited results. It’s a double edged sword because without good vessels it is hard to get medications to the tumor.
The new blood vessels grown by the cancer are different than ordinary brain vessels and are leaky. Leakiness in the tumor causes low oxygen, tissue damage, and fluid buildup (edema). Some therapies have attacked these new blood vessels with limited results. It’s a double edged sword because without good vessels it is hard to get medications to the tumor.
Another complication is that it isn’t clear how the vessels are produced. Both neuronal stem cells and tumor glioma stem cells can make vessel lining cells. Therefore, these vessels are unusual with different kinds of signals. In fact, when the brain cancer cells surround blood vessels they live in a stem cell niche, where stem cells for both the cancer and possibly the blood vessels co-exist. It is found that the capillaries send signals (nitric oxide) that increase these stem cells. Another example is astrocytes making a factor for the cancer and blood vessels (osteopontin).
Cancers in the brain use another technique for blood vessels. They take over the existing blood vessels in the organ and infiltrate them by surrounding cells and traveling along the route. This produces a microenvironment that is friendly to the cancer and its growth.
Astrocytes Do More for Cancer
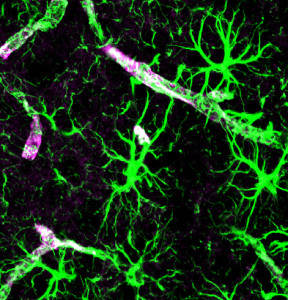 Astrocytes do more for cancer than at the blood brain barrier. With a large percentage of brain cells being astrocytes, they converse with cancer cells helping metastatic development. A previous post showed that both cancers and astrocytes use large vesicles called exosomes for sending vital genetic information to alter the environment. Astrocytes send microRNAs in exosomes that alter metastatic cancer cells in a way that helps them survive in the brain. These microRNAs alter genetic networks in the cell to lower a prominent gene that suppresses cancer (PTEN). This then increases growth of the cell through increasing a pathway PI3K. Surprisingly, this signal is directed at the particular cell not all the cells (for example not to local fibroblasts).
Astrocytes do more for cancer than at the blood brain barrier. With a large percentage of brain cells being astrocytes, they converse with cancer cells helping metastatic development. A previous post showed that both cancers and astrocytes use large vesicles called exosomes for sending vital genetic information to alter the environment. Astrocytes send microRNAs in exosomes that alter metastatic cancer cells in a way that helps them survive in the brain. These microRNAs alter genetic networks in the cell to lower a prominent gene that suppresses cancer (PTEN). This then increases growth of the cell through increasing a pathway PI3K. Surprisingly, this signal is directed at the particular cell not all the cells (for example not to local fibroblasts).
Astrocytes establish particular synapses with metastatic cancer cells. These synapses are the electrical gap junction type that sends electricity back and forth directly between the cells as signals. The cancer cell alters the astrocyte in this process as much as the opposite. The astrocyte then produces immune signals that increase inflammation to help the cancer (TNF) and specifically give the cancer growth factors. Metastasis has been decreased by stopping the development of gap junction synapses (disrupting connexin the vital molecule for gap junctions).
As seems to always be the case, there are a few opposite examples where astrocytes stop metastasis very early in the process.
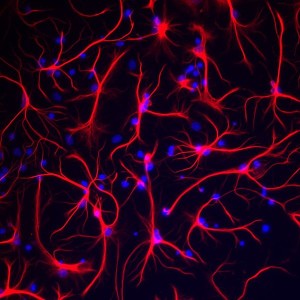
When injury occurs, astrocytes multiply to surround the area that is damaged, just as they cover the blood vessels. They work with microglia to continue to nourish neurons and axons to maintain the neuronal connections. This is called gliosis and many cytokines are produced in this process that form the brain’s version of inflammation. Just as inflammation helps cancers grow with the rich production of growth factors that can alter the normal regulatory inhibition on multiplication. Gliosis is another version of freewheeling reproduction and could help brain cancers. This also helps resistance to treatments, both blocking drugs input and creating new avenues of defense.
In newly launched metastatic colonies, gliosis also can help with factors to make the colony grow. The cancer cells start growing enzymes (serpins) which go against other enzymes made by the astrocytes to fight any new cancer cells. In some cases, the first treatment works against the cancer, such as blocking macrophages helping the cancer. Then when many are gone, other cells helping the cancer become stronger and fight back with a recurrence. These involve producing new signals and enzymes. Studies show that astrocyte reactions are vital to both killing cancers and helping them.
Neurons Help Cancers in the Brain and Outside
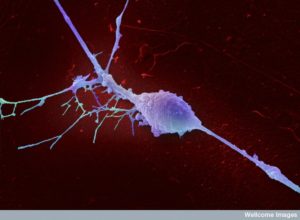 Neurons also produce factors that help the cancer. A previous post showed that neurons growing into many cancers are vital to the progression of the cancers and the travel of metastasis colonies. Recent studies in the brain show that neurons are involved in helping astrocytes grow and produce cancerous cells. Surprisingly, breast cancer cells that land in the brain start to produce neurotransmitters as if they are neurons using GABA. These neurotransmitters appear to help the cancer grow.
Neurons also produce factors that help the cancer. A previous post showed that neurons growing into many cancers are vital to the progression of the cancers and the travel of metastasis colonies. Recent studies in the brain show that neurons are involved in helping astrocytes grow and produce cancerous cells. Surprisingly, breast cancer cells that land in the brain start to produce neurotransmitters as if they are neurons using GABA. These neurotransmitters appear to help the cancer grow.
Matrix Connective Tissue
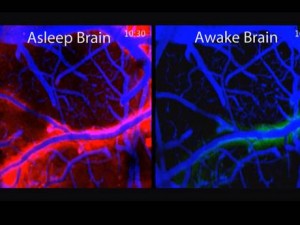
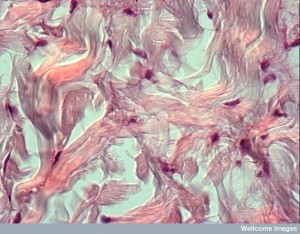 There is a very different structure of material in between cells in the brain than in other tissues. One aspect of this difference is the glymphatic cleaning system. Because it takes misfolded proteins out of the brain via a fluid flow between arteries, veins, and CSF, it needs more space between cells. This system includes brain tissues forming a lymphatic that connects to the CSF. An unusual aspect of this includes neurons shrinking in half each night to increase the flow and clean the brain. But for the neurons to shrink by half each night during sleep to encourage cleanout of debris, there must be space for this flow to occur.
There is a very different structure of material in between cells in the brain than in other tissues. One aspect of this difference is the glymphatic cleaning system. Because it takes misfolded proteins out of the brain via a fluid flow between arteries, veins, and CSF, it needs more space between cells. This system includes brain tissues forming a lymphatic that connects to the CSF. An unusual aspect of this includes neurons shrinking in half each night to increase the flow and clean the brain. But for the neurons to shrink by half each night during sleep to encourage cleanout of debris, there must be space for this flow to occur.
Brain matrix has less specific scaffolding from collagen and laminin, which are common in other tissues. These structural molecules are in the barriers surrounding blood vessels called the basement membrane, but not elsewhere. Other scaffolding molecules unique to the brain are large molecules combining protein and glycan carbohydrates. These are called glycoproteins and proteoglycans and form structures that produce the niche environments for stem cells and for blood vessels. As a previous post noted, capillary cells are intimately connected with stem cells with conversations about production of new cells and tissue.
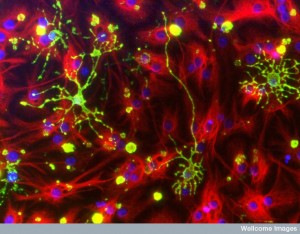 When gliosis occurs, there is a great amount of stem cell activity. This process can inadvertently produce factors that increase blood vessels locally and greatly help cancer cells. Gliosis also attracts macrophages that can also turn to help the cancers. Another side effect is that T cells have less access to the area and this limits T cell fighting with cancer and treatments using T cells. Some of the factors even work against T cells.
When gliosis occurs, there is a great amount of stem cell activity. This process can inadvertently produce factors that increase blood vessels locally and greatly help cancer cells. Gliosis also attracts macrophages that can also turn to help the cancers. Another side effect is that T cells have less access to the area and this limits T cell fighting with cancer and treatments using T cells. Some of the factors even work against T cells.
Recent studies show that the “stiffer” the matrix, the more cancer is produced. The cancer cells products increase the matrix stiffness.
Cancer Treatments Using Supportive Cells in the Brain
 Treatments are being developed using all of these avenues mentioned already—macrophages, DCs, vaccinations, anti-check point medications (anti PD-1), along with conventional drugs and X-rays and surgery. Future ideas include using neutrophils, influencing blood vessel production, altering gab junctions of astrocytes,
Treatments are being developed using all of these avenues mentioned already—macrophages, DCs, vaccinations, anti-check point medications (anti PD-1), along with conventional drugs and X-rays and surgery. Future ideas include using neutrophils, influencing blood vessel production, altering gab junctions of astrocytes,
What is needed however is more information about the complexities of the conversations going on in the environment among all of these different cells. Astrocytes can help avoid side effects of damage to brain tissues in their protective roles sometimes via forming gap junction synapses (direct two way electrical synapses) with cancer cells.
One new thought is that somehow treatments that aren’t supposed to get into the brain actually do seem to help, sometimes by accident. It is possible that the permeability is altered in various ways and that treatment for tumors outside of the brain that theoretically will not get to the tumor might actually help.
Supportive Brain Cells Help Cancers Via Communication
 As is true everywhere in the body—and in nature—it is the signaling conversations among cells that determines everything. As technological advances allow the observation of individual cell’s communication, more and more is learned. Cancers are very clever at gaining support of a wide range of supportive cells in each environment via conversations. A previous post showed how metastatic colonies take advantage of communication to gain the aid of all local cells in the environment.
As is true everywhere in the body—and in nature—it is the signaling conversations among cells that determines everything. As technological advances allow the observation of individual cell’s communication, more and more is learned. Cancers are very clever at gaining support of a wide range of supportive cells in each environment via conversations. A previous post showed how metastatic colonies take advantage of communication to gain the aid of all local cells in the environment.
The brain is a unique tissue with its own types of matrix, immune response, and anatomical structures. A previous post showed the many ways that neurons can help cancers establish themselves, grow, survive, and metastasize. It appears that all of the supportive cells in the brain also help cancers via conversations. As more is learned, future treatment will be based on these signals.