 Helping T cells and microbes kill cancers as new advanced treatment is hot news. These new treatments are based on the natural outcomes of communication among cells, including immune, lining, brain, and cancer cells along with microbes. It is definitely striking that all of these cells speak the same language and can talk to each other. T cells, as master regulators of the immune system, organize the attacks on invading microbes, inflammation, cancers, and any debris. T cells must adapt to each situation, but attacking cancer is unique in that it takes a long time, unlike microbe attacks that are severe and swift.
Helping T cells and microbes kill cancers as new advanced treatment is hot news. These new treatments are based on the natural outcomes of communication among cells, including immune, lining, brain, and cancer cells along with microbes. It is definitely striking that all of these cells speak the same language and can talk to each other. T cells, as master regulators of the immune system, organize the attacks on invading microbes, inflammation, cancers, and any debris. T cells must adapt to each situation, but attacking cancer is unique in that it takes a long time, unlike microbe attacks that are severe and swift.
When the T cells are not able to function properly, cancers can flourish and this involves special communication among all of the cells, such as caners involving local structural cells in their cause. Recent research has begun to understand some of these conversations and they appear to be different in chasing cancers and in dealing with the many kinds of inflammation. This post reviews the unique cellular communication related to T cells and their response to cancers.
The life of T cells is complex and each responds differently in many varied circumstances, producing unique versions of a wide variety of different kinds of T cells for each situation. Each type of T cell uses various different signals—for allergies, microbe infections, trauma, autoimmune responses, and response to cancers.
 One major immune response to viruses and those bacteria that invade human cells is called type 1 immune responses. The special T cells produced in this situation are CD8+T cells and CD4+ helper T cells, which can kill those cells that are infected. These two types of cells, sometimes called antitumor T cells, along with T helper 1 (Th1) cells are, also, used in the unique attack against cancers. This type of response is very old in evolution and has become specialized for the vast number of different types of microbe attacks that have occurred over a long period of time. It, also, evolved with responses to different cancers.
One major immune response to viruses and those bacteria that invade human cells is called type 1 immune responses. The special T cells produced in this situation are CD8+T cells and CD4+ helper T cells, which can kill those cells that are infected. These two types of cells, sometimes called antitumor T cells, along with T helper 1 (Th1) cells are, also, used in the unique attack against cancers. This type of response is very old in evolution and has become specialized for the vast number of different types of microbe attacks that have occurred over a long period of time. It, also, evolved with responses to different cancers.
Common Response to Infections has Programmed Inhibition
 Type 1 responses include multiple different ways that the attack on microbes will decrease rapidly because if continued then damage to human tissues will occur through immune related disease. This inhibition of T cell activity, however, is a disadvantage in fighting cancers, which continue on as a threat for a long time. In fact, cancer cells themselves take advantage of these inhibitory signals.
Type 1 responses include multiple different ways that the attack on microbes will decrease rapidly because if continued then damage to human tissues will occur through immune related disease. This inhibition of T cell activity, however, is a disadvantage in fighting cancers, which continue on as a threat for a long time. In fact, cancer cells themselves take advantage of these inhibitory signals.
Along with cancer cells inhibiting T cells through signaling, they recruit other cells in their micro environment. Many cells contribute to decreasing the immune attack on cancers, much to the cancer’s advantage. These include the cancer cells themselves, structural tissue cells (stromal cells) that have been influenced by the cancers to become allies, and immune cells producing many cytokine signals. All of these cells become a team, signaling each other and supporting a decrease in T cell attacks on the cancer.
 The many cytokines produced by all of these cells have metabolites that increase the number of active molecules contributing to the complex environment that the cancer uses to grow its unique colony. It is metabolism products of all of these cells that alter T cell functions and these changes are often based on the food available to these cells producing different signals. These signals alter the T cells movements, their strength and ability to survive, whether they will multiply, and specific methods of attack. All of these functions are highly regulated in the complex T cells.
The many cytokines produced by all of these cells have metabolites that increase the number of active molecules contributing to the complex environment that the cancer uses to grow its unique colony. It is metabolism products of all of these cells that alter T cell functions and these changes are often based on the food available to these cells producing different signals. These signals alter the T cells movements, their strength and ability to survive, whether they will multiply, and specific methods of attack. All of these functions are highly regulated in the complex T cells.
Cancer cells gain strength and persist despite attacks through communication with other cells by molecular signals, direct contact, and sharing or withholding available food. It is these many types of cellular conversations that form the basis of the most advanced cancer treatment.
Regulatory T Cells
 There is a complex balance of active killer T cells and T cells that regulate the environment (T reg cells). These have been described in previous posts as vital to autoimmune disease, food allergies, and a variety of different infections. These are absolutely critical in the response to cancer. In fact, regulatory T cells can become the dominant type of T cell in the cancer environment (CD4+ T cells).
There is a complex balance of active killer T cells and T cells that regulate the environment (T reg cells). These have been described in previous posts as vital to autoimmune disease, food allergies, and a variety of different infections. These are absolutely critical in the response to cancer. In fact, regulatory T cells can become the dominant type of T cell in the cancer environment (CD4+ T cells).
Some regulatory T cells respond to signals, such as transcription factors, and suppress CD8+ cells. These Treg cells stop cells from being activated to attack cancer, stop new cytokine production, and slow reproduction. They use several important cytokines that work against inflammation—transforming growth factor-β (TGFβ) and interleukin-10 (IL-10). Working against these regulatory cells strengthens the attack on cancers. The factors that determine these outcomes appear to be the metabolic activity going on in the neighborhood of the cancer.
Unusual Activity Near Cancer Environments
 Normally, a class of cells pick up abnormal antigens and present them to T cells. These are called APCs for antigen presenting cells and are necessary to activate killer T cell activity against microbes and cancers. Dendritic cells of many types are vital APCs. But, surprisingly in the small region around cancer cells there are few of these and the ones that are present have less of the vital proteins in the membranes that help stimulate killer cells (proteins CD80 and CD86). In fact, at the edges of the cancers, there are abnormal APCs that do the opposite of what they are supposed to do by stopping killer T cells. Another class of cells enters the cancer milieu and suppresses T cells as well by nitric oxide, reactive oxygen metabolic products, and other metabolites. All of these are now targets of cancer treatments to re activate the T cells to attack.
Normally, a class of cells pick up abnormal antigens and present them to T cells. These are called APCs for antigen presenting cells and are necessary to activate killer T cell activity against microbes and cancers. Dendritic cells of many types are vital APCs. But, surprisingly in the small region around cancer cells there are few of these and the ones that are present have less of the vital proteins in the membranes that help stimulate killer cells (proteins CD80 and CD86). In fact, at the edges of the cancers, there are abnormal APCs that do the opposite of what they are supposed to do by stopping killer T cells. Another class of cells enters the cancer milieu and suppresses T cells as well by nitric oxide, reactive oxygen metabolic products, and other metabolites. All of these are now targets of cancer treatments to re activate the T cells to attack.
Special macrophages are also produced that would normally repair tissue, and here they also help the cancers. These M2 macrophages stop the anti-cancer immune response 1. These help in other ways such as stopping the normal regulation of building blood vessels. These are also helped by particular metabolic molecules that signal for these pro tumor anti T cell effects. Increased acid and decreased oxygen help these processes. The opposite M1 macrophages support T cells to fight cancers. They destroy stromal or structural tissue cells that have already aligned with the cancer, and they stop excess blood vessels.
 The local structural tissue cells such as fibroblasts and fat cells are recruited and altered by the cancer in the local neighborhood. They help the tumor with more matrix and special cytokines that build a cancer friendly architecture. They produce other special signals to suppress T cells as well—such as VEGF or vascular endothelial growth factor. Fat cells help breast and ovarian cancers. They allow invasion by cancer cells and fight T cells.
The local structural tissue cells such as fibroblasts and fat cells are recruited and altered by the cancer in the local neighborhood. They help the tumor with more matrix and special cytokines that build a cancer friendly architecture. They produce other special signals to suppress T cells as well—such as VEGF or vascular endothelial growth factor. Fat cells help breast and ovarian cancers. They allow invasion by cancer cells and fight T cells.
Blood vessel lining cells help build larger cancers that require new blood vessels to grow. These vessels become quite abnormal without the normal patterns of adhesion or the second layer of pericytes (see post). They inhibit the cytokines that call for T cells to fight the cancer, such as by producing prostaglandin E2 (PGE2). Even lymphatic vessel lining cells get into the act and help the cancers.
Metabolic Signals in the Environment Near the Cancer
 A previous post showed how T cells use ordinary metabolic pathways as signals to support their extraordinary stimulation, where suddenly cells can grow large and reproduce into a large army of fighting cells. Cancer cells have learned how to use the same metabolic signals to trigger their exponential growth. These ordinary metabolic pathways include glycolysis, amino acid metabolism, and fatty acid production. T cells rely on these pathways as signals for their activity.
A previous post showed how T cells use ordinary metabolic pathways as signals to support their extraordinary stimulation, where suddenly cells can grow large and reproduce into a large army of fighting cells. Cancer cells have learned how to use the same metabolic signals to trigger their exponential growth. These ordinary metabolic pathways include glycolysis, amino acid metabolism, and fatty acid production. T cells rely on these pathways as signals for their activity.
Cancer cells copy this and compete with the T cells for materials and nutrients in the neighborhood. Caners compete for sugar with T cells and impair T cell functions directly. Amino acids are also vital to both to grow large and multiply. They damage T cells because of less available arginine amino acid.
 Eating plenty of glutamine helps cancers grow. T cells target the enzyme for glutamine, gluaminase, which stops cancers from growing. Many of the factors mentioned already help the cancers in this competition. By taking lactate from the T cell, cancers further stop T cell activity. The fight for tryptophan is another way cancers help their cause.
Eating plenty of glutamine helps cancers grow. T cells target the enzyme for glutamine, gluaminase, which stops cancers from growing. Many of the factors mentioned already help the cancers in this competition. By taking lactate from the T cell, cancers further stop T cell activity. The fight for tryptophan is another way cancers help their cause.
Adenosine and PGE2 (prostaglandin) help cancers in this way as well. PGE2 is produced in infections that last and impair signaling in T cells (by taking up protein kinase A). Therapy aimed at PGE2 has helped some cancers by stimulating T cells. The effect of adenosine is similar.
Abnormal fatty acids hurt T cell activity against cancers. Tumors produce a lot of fatty acids with abnormal fat cells and other support cells.
Inhibiting T Cells
 There are many ways that T cells are inhibited. Some are vital mechanisms that evolution has produced to protect against too much inflammation. Others are produced by alterations in genetic networks (mutations). Recent research shows that cancer cells are filled with genetic mutations, including many different ones in each individual patient’s cancers, but also there are major variations of mutations within regions of the same cancers.
There are many ways that T cells are inhibited. Some are vital mechanisms that evolution has produced to protect against too much inflammation. Others are produced by alterations in genetic networks (mutations). Recent research shows that cancer cells are filled with genetic mutations, including many different ones in each individual patient’s cancers, but also there are major variations of mutations within regions of the same cancers.
There are genes that help produce cancers (oncogenes) and the opposite (tumor suppressor genes). Some of these mutations help inhibit T cells. Melanoma activates a pathway that stops dendritic cells that normally stimulate T cells to fight cancers. Several particular mutations (BRAF and PTEN) stimulate cytokines that stop T cells (IL-1 and VEGF). Ovarian cancer makes signals that decrease T cells by epigenetic tag mechanisms. These mutations that affect T cells are about 10% of the patients.

But, cancers cells don’t really need these mutations to stop T cells. The inhibition of the type 1 immune response is, in fact, very common. Cancers manipulate both the innate and adaptive mechanisms of immune defense. These types of responses can affect a wide range of different cancers. In fact, they occur whenever there is long standing inflammation or infection.
These natural inhibition mechanisms must occur after any infection or damage with result, even if the microbes are not fully destroyed. In fact, the remaining microbes are often much less damaging than continued attacks by the powerful T cells. One of the major inhibitory cytokines are type 1 interferons (IFNs) either naturally are given as treatments. Strikingly, even attacking T cells such as CD8+ can produce one of these IFNs.
T cell Exhaustion and Continued Battle Against Cancers
 After long infections, T cells were described as exhausted. Another group of T cells were called “senescent” (can’t divide anymore) or “anergic” (don’t respond to stimulation with signals). But, recent research shows that T cells still function against cancers but in different and perhaps more subtle ways. They are not really exhausted but rather they are now called a different “long term T cells”. T cell changes in cancer neighborhoods are not as well researched and the properties of this new long term cancer fighting T cell is just being found.
After long infections, T cells were described as exhausted. Another group of T cells were called “senescent” (can’t divide anymore) or “anergic” (don’t respond to stimulation with signals). But, recent research shows that T cells still function against cancers but in different and perhaps more subtle ways. They are not really exhausted but rather they are now called a different “long term T cells”. T cell changes in cancer neighborhoods are not as well researched and the properties of this new long term cancer fighting T cell is just being found.
The new term has been “dysfunctional” to say that the T cell is acting differently, but still subtly against the cancer, but withou the strong reaction that damages local tissues by excessive inflammation. Anergic T cells don’t work properly at all. But these vital T cells against cancer have unique metabolic changes that use normal responses to T cells in different ways. They don’t become armies of killer cells through metabolic change, but still fight the cancers with less local disturbance.
With low sugar, special genes are triggered in an unusual manner. Using cytokines against inflammation and many transcription factors (IL-10 and IL-6), they have complex pathways that are different in those that will go after the cancer and invade the tissue.

Another specific set of genes in CD8+ T cells that invade cancers have been recently found. These genes appear to respond in different ways at the beginning of the infection and then later. Also, they respond differently in cancer invasion. After response to infections, the amount of microbes decreases and the activity is altered. They modulate activity without becoming overwhelming—a modified attack. When T cells are near the “exhausted” state they modulate specific functions. Multiple different pathways modify the cancer attacking T cell. They are just being discovered and will become part of new advanced treatments.
Many Mechanisms for Long Term T Cells
Special T cell receptors that are normally used for inhibition produce a wide variety of different proteins. Special proteins are produced in the cell membrane. Special transcription factors are produced as well as interleukin 10. New molecules that can kill cancer cells are produced such as Granzyme B.
All of these changes usually occur after a week of infection and then subside. But, in the cancer fighting T cells, some of these changes last and others don’t. Many special molecules are produced only for cancer cells in CD8+. These includes multiple cytokines related to cellular navigation (CCL3, CCL4, CCL5). They include unique molecules on the surface membranes such as CD7, CD69. They include proteins, which are part of unusual receptors and transcription factor proteins. Other unique modifying factors are related to signaling inhibition.
 All of these special processes are just recently discovered in the cancer fighting T cells that maintain a long term attack. These changes are must less obvious or radical as the killer T cells, but does provide a sustained attack by some cells against the cancers.
All of these special processes are just recently discovered in the cancer fighting T cells that maintain a long term attack. These changes are must less obvious or radical as the killer T cells, but does provide a sustained attack by some cells against the cancers.
Very recent research has found that specific genes activate anti-cancer T cells triggered by particular cancers such as melanoma. This research shows that one particular transcription factor produces many different pathways that fight against cancer. These factors interact with the many factors mentioned above in the cancer milieu.
A separate specific T cell has been found in ovarian and breast cancers. This involves completely different pathways and effects than the melanoma pathway. Factors might not just be from different cancers, but even different individuals with the same cancer.
Advanced Therapy Using T Cells
 Advanced research into cancer therapies involves both stopping the inhibition of T cells and increasing the responses of anti-cancer T cells. It is complicated, however. Increasing activation of T cells can trigger more inhibitory responses. The best therapies have been decreasing inhibition of T cells through many different mechanisms, because the activation responses are not as strong. In some ways specific targeting of mutations that increase inhibition might be best.
Advanced research into cancer therapies involves both stopping the inhibition of T cells and increasing the responses of anti-cancer T cells. It is complicated, however. Increasing activation of T cells can trigger more inhibitory responses. The best therapies have been decreasing inhibition of T cells through many different mechanisms, because the activation responses are not as strong. In some ways specific targeting of mutations that increase inhibition might be best.
The battle against cancer is waged on many fronts at once and must go on for a long time in a way that allows the T cell to survive. As well as the many specific changes listed above, T cells have alterations that are specific to particular cancers and even individuals.
Immune Memory
 Immune cells have a memory of particular problems based on specific antigen molecules and this is the basis of a continued battle against cancers over a long period of time, rather than the several week attacks for microbes. Recent immune therapies against even metastatic cancers have been increasingly successful, but still not in the majority of cases. Specific metabolic products are useful for both activation and stopping T cell inhibition from regulatory cells.
Immune cells have a memory of particular problems based on specific antigen molecules and this is the basis of a continued battle against cancers over a long period of time, rather than the several week attacks for microbes. Recent immune therapies against even metastatic cancers have been increasingly successful, but still not in the majority of cases. Specific metabolic products are useful for both activation and stopping T cell inhibition from regulatory cells.
The best approach seems to be stopping the inhibition by blocking specific inhibitory receptors, getting rid of certain metabolites, and stopping M2 macrophages, and other regulatory T cells. The specific mechanisms that are causing inhibition of T cell activity are called “checkpoints” and the therapy of attacking these specific mechanisms are called “checkpoint therapies” or “checkpoint blockade”. Even when mutations have increased T cell inhibition, these strategies work with or without attack on the mutation effects. Blocking the CTLA4 inhibitory receptor has helped greatly with several solid cancers. There are several other identified inhibitory receptors that might also work (TIM3, LAG3, TIGIT).
 In some cancers the combination of drugs that stimulate T cells and those that target the blockade are best.
In some cancers the combination of drugs that stimulate T cells and those that target the blockade are best.- Other therapies attack the particular tissue stromal cells that are blocking T cells entry into the cancer milieu. One technique blocks a factor that stimulates macrophages and others that stimulate M1 types.
- Some therapies take T cells from the patient, alter them and infuse them back.
- Some place new receptors that stimulate T cells based on particular molecules (antigens) from the particular cancer.
- Other attempts place receptors that will help find hidden cancer cells.
- Some are attempting to alter proteins in T cells to help them resist inhibition.
- Some try to change the genetic network to make them behave more like memory cells. Metabolism is very complex and it is not yet clear which alterations will help.
- Some take out inhibitory receptors. So far, activators are not helpful because they trigger too many responses. These researchers are looking for more limited specific factors such as STAT5, which stimulates killer activity.
- Many of these techniques rely on CRISPR-Cas9 clustered, regularly interspaced palindromic repeat–CRISPR- associated protein 9).
The Fight Goes On
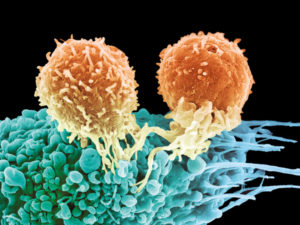 T cells can help fight cancer if they can keep their status as a type 1 immune response but for a long time. This occurs naturally in a mild way, but therapies can increase the effectiveness and continue the fight against cancers. Normally, the strength of the killer T cell lasts several days before it decreases. Repeated stimulation is necessary or decrease of the many inhibitory regulatory mechanisms. T cell memory cells are the best at renewing themselves and continuing to monitor and fight a particular problem. Memory T cells also maintain the ability to rapidly become killer cells. The details of memory cells related to chronic infections and cancer is just now being looked into.
T cells can help fight cancer if they can keep their status as a type 1 immune response but for a long time. This occurs naturally in a mild way, but therapies can increase the effectiveness and continue the fight against cancers. Normally, the strength of the killer T cell lasts several days before it decreases. Repeated stimulation is necessary or decrease of the many inhibitory regulatory mechanisms. T cell memory cells are the best at renewing themselves and continuing to monitor and fight a particular problem. Memory T cells also maintain the ability to rapidly become killer cells. The details of memory cells related to chronic infections and cancer is just now being looked into.
In fact, it is probable that a small proportion of the remaining T cells in the cancer milieu are memory cells and continue to produce fighting cells. But, finding them is difficult for researchers. These memory T cells may reside in lymph nodes, not even near the cancer.
T Cells Adapt to Fight Cancer
 There is substantial research about T cells in lymph tissue, but only recently has research looked at T cells in the micro environment of cancers. In these micro environments stromal cells include fat cells, neurons, dendritic cells, fibroblasts, pericytes, capillaries. All of these types of cells can be altered to work for the betterment of the cancer community. This occurs through signaling between the cells.
There is substantial research about T cells in lymph tissue, but only recently has research looked at T cells in the micro environment of cancers. In these micro environments stromal cells include fat cells, neurons, dendritic cells, fibroblasts, pericytes, capillaries. All of these types of cells can be altered to work for the betterment of the cancer community. This occurs through signaling between the cells.
Cancer cells live as a community with constant communication among themselves and with all of the local cells that they are attracting to help them. When Cancer cells are attacked by T cells, they respond with altered signals and communications. Despite the great complexity of these responses from different individuals, tissues, different cancers, different cells in segments of cancers, researchers are learning the signaling language and pathways to devise new ways to attack the cancer. One of the best methods turns out to help T cells survive in their slow subtle attack on cancers and their supportive cells.
These are complex examples of the language of cells, and the advanced cancer treatments will be intercepting this natural communication among cells.
 In some cancers the combination of drugs that stimulate T cells and those that target the blockade are best.
In some cancers the combination of drugs that stimulate T cells and those that target the blockade are best.