 It has become clear that all brain processes are extremely dynamic and ever changing. Neurons alter themselves every day including elaborate reworking of the scaffolding for long axons and new dendrites. At synapses, neuroplasticity alters synapses in many different ways (see post). For many years, it was assumed that there were few to no immune cells in the brain. This was called “immune privilege”. But, now it is clear that there are a large number of immune cells in the brain both the resident type called microglia, as well as cells from the blood that travel through the brain for various purposes. Previous posts noted how a small army of T cells exist in the brain and CSF. They signal to the brain cells constantly. They signal tonic messages to maintain cognition. With acute stress, they send signals to increase memory and learning. And when there is an infection and a need to rest, they send signals to the brain to slow down and limit cognition and activity—the “sick feeling”.
It has become clear that all brain processes are extremely dynamic and ever changing. Neurons alter themselves every day including elaborate reworking of the scaffolding for long axons and new dendrites. At synapses, neuroplasticity alters synapses in many different ways (see post). For many years, it was assumed that there were few to no immune cells in the brain. This was called “immune privilege”. But, now it is clear that there are a large number of immune cells in the brain both the resident type called microglia, as well as cells from the blood that travel through the brain for various purposes. Previous posts noted how a small army of T cells exist in the brain and CSF. They signal to the brain cells constantly. They signal tonic messages to maintain cognition. With acute stress, they send signals to increase memory and learning. And when there is an infection and a need to rest, they send signals to the brain to slow down and limit cognition and activity—the “sick feeling”.
Inflammation was also considered to be abnormal in the brain. It is now known that there are many different kinds of inflammation, including a type stimulated by neurons as part of neuroplasticity—one definition of neuro inflammation. Recent research shows that abnormal types of inflammation with alterations in microglia and astrocytes are part of states that increase degenerative brain disease.
 Now it is clear that there are types of inflammation that are helpful—fighting infection and healing tissues—and there are types of inflammation that increase damage over time. It has not been clear how various types of inflammation affect traumatic brain injury (TBI).
Now it is clear that there are types of inflammation that are helpful—fighting infection and healing tissues—and there are types of inflammation that increase damage over time. It has not been clear how various types of inflammation affect traumatic brain injury (TBI).
When the brain is injured, many processes attempt to heal the damage. Other processes can possible increase damage over time. One major cellular mechanism to get rid of damaged molecules and debris is called autophagy. This is a complex process whereby large vesicles eliminate debris in sync with lysosomes—large vesicles with machinery to take apart molecules. But, autophagy is also part of many other extremely complex cellular pathways with a variety of functions.
Autophagy is a very ancient way that eukaryotic cells keep cells clean. There are elaborate cargo transport mechanisms for debris. There are mechanisms that involve the chaperone molecules that fold proteins in the endoplasmic reticulum. There are large special vesicles that sequester parts of cells and microbes to be destroyed. These pathways are triggered in relation to general metabolic cycles and signals related to mTOR (see post).
Recently, it has been found that autophagy operates differently in different situations that occur with brain problems. In acute neurotoxicity from poisons and various drugs, autophagy helps with positive cleanup of damage. In several other circumstances it increases damage. These include strokes in adults, excitotoxicity from excessive glutamate, and neonatal asphyxia.
But, in traumatic brain injury (TBI) the research has not been clear whether these pathways are positive or negative.
TBI and Inflammation
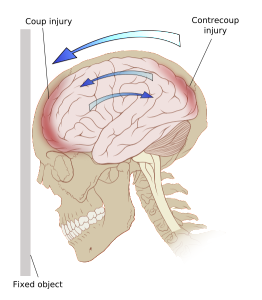
At first TBI was considered to be static with no increased damage over time. While TBI might be associated with more dementias, brain injuries were considered independent of additive events. With recent research related to degenerative disease, there is some evidence that specific forms of brain injury increases brain degeneration. Now, there is a question whether inflammation has the same possible negative effects on TBI.
TBI’s definitely produce inflammation response in the brain as any injury would. These include cells from the blood coming into the brain as well as resident immune cells such as microglia in conjunction with other glia. This inflammation can last for many years even from one injury. There is some evidence that further brain degeneration might occur after TBI related to inflammation. Treatment with medications that stop inflammation have not been helpful in research trials.
Positive Inflammation After Acute Injury
 Now, it appears that some of the effects of inflammation after TBI can be helpful to the healing of the brain, especially before the effects become chronic. It appears that the inflammation just after the TBI can influence the cells needed to repair the damage. This is probably why suppressing inflammation after TBI may be harmful instead of helpful. There is a question as to whether inflammation stops neurons from regenerating. It has been recently learned that neuro inflammation caused by neurons can be part of neuroplastic changes and can be helpful.
Now, it appears that some of the effects of inflammation after TBI can be helpful to the healing of the brain, especially before the effects become chronic. It appears that the inflammation just after the TBI can influence the cells needed to repair the damage. This is probably why suppressing inflammation after TBI may be harmful instead of helpful. There is a question as to whether inflammation stops neurons from regenerating. It has been recently learned that neuro inflammation caused by neurons can be part of neuroplastic changes and can be helpful.
Acute damage in the brain causes quite a stir. Responses come from microglia and other macrophages outside of the brain—microglia are in the macrophage family but traveled to the brain in the fetus and never left. Responses also are from other blood cells including T cells, neutrophils and monocytes.
Microglia pick of damage pattern signals (DAMPs – damage associated molecular patterns – see posts on pattern recognition receptors). This stimulates many cytokine signals, which changes the local environment to allow blood cells to enter.
Damaging Inflammation
 Alterations in microglia can last for a long time. Research shows altered (activated) microglia were present in a quarter of patients after a year. They also have damaged axons. Minocycline inhibits microglia and helps a little with reduced size of damage. But, these results are not certain. it is complicated by the fact that minocycline has many other effects then on microglia. Another study linked microglia to increased reactive oxygen species (ROS), which is a metabolic product made of oxygen that is very chemically active and can therefore cause damage. But, this was also not necessarily specific to microglia.
Alterations in microglia can last for a long time. Research shows altered (activated) microglia were present in a quarter of patients after a year. They also have damaged axons. Minocycline inhibits microglia and helps a little with reduced size of damage. But, these results are not certain. it is complicated by the fact that minocycline has many other effects then on microglia. Another study linked microglia to increased reactive oxygen species (ROS), which is a metabolic product made of oxygen that is very chemically active and can therefore cause damage. But, this was also not necessarily specific to microglia.
Monocytes from the blood produce many macrophages at the damage site. Although they are supposed to help heal, they appear to cause damage as well. They were shown to increase damage to the hippocampus after injury to the cortex.
These findings triggered many studies with medications to inhibit immune responses. Some of the early trials showed some improvement. Steroids have been used for acute brain injury for a generation in emergency rooms. A large study in acute brain injury in 204 showed that if given in the first 8 hours, steroids increased the amount of death by the sixth month after the TBI. The group given steroids also were less likely to improve. A second large study using progesterone, which is not a steroid and fights inflammation in the first 4 hours after TBI. There was no improvement at six months.
 These studies show that very early on, it is not good to stop the inflammation. Some inflammation might be necessary to increase healing and regeneration. When thinking about inflammation outside of the brain, it is known that inflammation is part of wound healing by bringing appropriate cells to clean out debris, create a barrier for the healing work, and then helping healing. So, it is reasonable at this point to consider that T cells, microglia, macrophages, and monocytes can help maintain and improve regeneration.
These studies show that very early on, it is not good to stop the inflammation. Some inflammation might be necessary to increase healing and regeneration. When thinking about inflammation outside of the brain, it is known that inflammation is part of wound healing by bringing appropriate cells to clean out debris, create a barrier for the healing work, and then helping healing. So, it is reasonable at this point to consider that T cells, microglia, macrophages, and monocytes can help maintain and improve regeneration.
Microglia are the first responders and pick up the first DAMPs. They respond rapidly to purines like ATP not inside cells, where they usually are. They immediately send their arms towards the damage. In one study of trauma to meninges, ATP was released by astrocytes and this immediately changes the shape of microglia into looking like a jelly fish. This altered shape of the microglia helps create a barrier around the damaged area to support repair. It also helps with clearance of debris by eating it (microglia are essentially phagocytes when they are not being a brain cell). If this microglia responses was inhibited, then many more neurons died.
When capillaries are damaged causing bleeding, microglia arms created a barrier to stop the bleeding further. When a laser was used to make a very small exact lesion, microglia sent arms right to the spot to stop any leaking from the vessels. When microglia were suppressed, the blood loss was three times as long and much more damaging. In another study where the corpus callosum was damaged, microglia were necessary to stimulate the stem cells that make oligodendrocytes to make more myelin. Microglia eat the defective myelin, but when they can’t signal to the oligodendrocyte stem cells, the myelin production becomes chaotic.
All of this research is consistent with microglia being vital in the acute phase of the TBI, especially creating barriers for blood loss and eating debris. But, they actually do more.
 Although normally, it is difficult for immune cells to enter the CSF and brain tissue, they can with back and forth signaling to the guardian cells of the blood brain barrier (pericytes, capillaries and astrocytes) and the blood cerebrospinal fluid barriers (the choroid cells). With damage, immune cells much more rapidly come into the brain. In fact, neutrophils were shown to be necessary for healing. Without neutrophils, lesions increase, neurological symptoms were worse and astrocytes became defective. Neutrophils travelling to the meninges increased survival of the meningeal cells. Although it is not yet known what signals are used by neutrophils their importance for healing and better outcomes is clear. One thing neutrophils do is to call for monocytes from the blood. These later cells become wound healing macrophages several days after the injury.
Although normally, it is difficult for immune cells to enter the CSF and brain tissue, they can with back and forth signaling to the guardian cells of the blood brain barrier (pericytes, capillaries and astrocytes) and the blood cerebrospinal fluid barriers (the choroid cells). With damage, immune cells much more rapidly come into the brain. In fact, neutrophils were shown to be necessary for healing. Without neutrophils, lesions increase, neurological symptoms were worse and astrocytes became defective. Neutrophils travelling to the meninges increased survival of the meningeal cells. Although it is not yet known what signals are used by neutrophils their importance for healing and better outcomes is clear. One thing neutrophils do is to call for monocytes from the blood. These later cells become wound healing macrophages several days after the injury.
 T cells are also important in injury (the delayed response from the adaptive immune system rather than the immediate innate immune system), even though they are usually associated with fighting microbes and cancers. T cells send special CD4+ T cells that produce interleukin-4. This cytokine is able to stimulate factors that are needed for neuronal growth. These CD4+ work with other special T cells that perform other jobs. Regulatory T cells also regulate the inflammation and stop it before it becomes dangerous in other situations (see post). In this situation these regulatory T cells are inhibited so that more inflammation and healing can occur. This actively promotes the healing aspect of immune cells’ work. In research without these regulatory T cells, macrophages were able to change into the wound healing types from their inflammation types.
T cells are also important in injury (the delayed response from the adaptive immune system rather than the immediate innate immune system), even though they are usually associated with fighting microbes and cancers. T cells send special CD4+ T cells that produce interleukin-4. This cytokine is able to stimulate factors that are needed for neuronal growth. These CD4+ work with other special T cells that perform other jobs. Regulatory T cells also regulate the inflammation and stop it before it becomes dangerous in other situations (see post). In this situation these regulatory T cells are inhibited so that more inflammation and healing can occur. This actively promotes the healing aspect of immune cells’ work. In research without these regulatory T cells, macrophages were able to change into the wound healing types from their inflammation types.
These studies show that there is a critical dynamic in an injury from the immediate response, to several days, to longer.
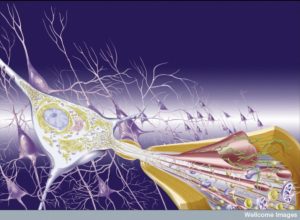 Recent research is consistent with many different kinds of inflammation (see other posts on degenerative brain injury and maternal immune activation). Also, there are many different dynamic changes after an acute brain injury. Inflammation appears to be essential immediately after the injury. A different kind of inflammation is necessary several days later. Different jobs take precedence—barrier creation and maintenance, clearing of debris, production of cytokines to stimulate repair, production of special factors that neurons need for growth, and regulation of the complex processes occurring. All of this includes coordination with many different kinds of cells through communication.
Recent research is consistent with many different kinds of inflammation (see other posts on degenerative brain injury and maternal immune activation). Also, there are many different dynamic changes after an acute brain injury. Inflammation appears to be essential immediately after the injury. A different kind of inflammation is necessary several days later. Different jobs take precedence—barrier creation and maintenance, clearing of debris, production of cytokines to stimulate repair, production of special factors that neurons need for growth, and regulation of the complex processes occurring. All of this includes coordination with many different kinds of cells through communication.
If any of these processes is interfered with, more damage will occur.
But, there is also no doubt that over time, other negative types of inflammation can occur and spread the damage. It can even connect damage with other degenerative brain disease processes. This can occur when microglia and macrophages are left for months or years to deal with the damage. They can change into different shapes and functions related to increasing inflammation again of the damaging variety.
Update on Traumatic Brain Injury and Inflammation
 Research into the effects of different types of inflammation after traumatic brain injury are complex. Some appear to be essential for recovery and others appear to spread damage and even merge with degenerative brain disease. There are many variables that can affect these processes—genetics, toxins, environments, and special locations of the injury. The severity can also be significant.
Research into the effects of different types of inflammation after traumatic brain injury are complex. Some appear to be essential for recovery and others appear to spread damage and even merge with degenerative brain disease. There are many variables that can affect these processes—genetics, toxins, environments, and special locations of the injury. The severity can also be significant.
As mentioned above, the general process of autophagy has many different responses as well. In some brain diseases autophagy is very helpful and in others it is damaging. While research has clearly shown that some types of damage produce positive effects from autophagy and some negative, with TBI it is not clear. This is possibly because there is a sequence of events with different results at different times.
Everything in biology is based on communication among cells. The very complex set of players in response to brain injury and the varied goals of their activities make it difficult to understand all of this signaling. But, somehow it is all coordinated between neurons, glia and immune cells from outside of the brain.