 Immune cells travel independently and depend on signals for their activity. Called the “wireless” brain, immune cells communicate with many other cells—neurons, astrocytes, microglia, blood vessel cells, intestinal and skin lining cells, and tissue cells. Signals help develop special capabilities, such as T cells controlling food reactions (see previous post). Signals maintain immune cells for many years as memory sentinels for particular infections. Signals tell where trouble is occurring and guide cells across difficult terrain to locations of inflammation and trauma.
Immune cells travel independently and depend on signals for their activity. Called the “wireless” brain, immune cells communicate with many other cells—neurons, astrocytes, microglia, blood vessel cells, intestinal and skin lining cells, and tissue cells. Signals help develop special capabilities, such as T cells controlling food reactions (see previous post). Signals maintain immune cells for many years as memory sentinels for particular infections. Signals tell where trouble is occurring and guide cells across difficult terrain to locations of inflammation and trauma.
Immune cell communication includes secreted molecules of all kinds and elaborate sets of information molecules in vesicles. Recently, the importance of micro RNA signals has been discovered. Research shows that immune micro RNA signals are vital for most immune functions. Micro RNA interact with all other signals such as cytokines, neurotransmitters, lipids, sugars and peptides. This post will review what is being found in these incredibly complex overlapping communication systems and the vast complexity of immune micro RNA signals.
Making New Blood Cells
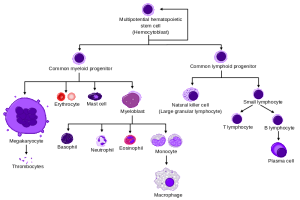 In order to make various blood cells, genetic mechanisms are highly regulated, but not totally understood. All blood cells appear to come from lines originating with one stem cell called the long term haematopoietic stem cells (LT-HSC). These long-term cells reproduce themselves and make other more specific stem cells called short term stem cells (ST-HSC). Short-term stem cells make limited stem cells (called multi potent progenitors or MPP) that can make a small variety of different blood cells. These stem cells produce even more limited stem cells that make either myeloid cells (common myeloid progenitors (CMP) or lymph cells (common lymphoid progenitors or CLP).
In order to make various blood cells, genetic mechanisms are highly regulated, but not totally understood. All blood cells appear to come from lines originating with one stem cell called the long term haematopoietic stem cells (LT-HSC). These long-term cells reproduce themselves and make other more specific stem cells called short term stem cells (ST-HSC). Short-term stem cells make limited stem cells (called multi potent progenitors or MPP) that can make a small variety of different blood cells. These stem cells produce even more limited stem cells that make either myeloid cells (common myeloid progenitors (CMP) or lymph cells (common lymphoid progenitors or CLP).
The many different immune and blood cells are needed for different circumstances. Short or chronic stress stimulates particular cells. Long-term stem cells must survive inflammation, illness and aging. Stem cells are directed by signals of many types and epigenetic tags. Non-coding RNAs are vital signals in this process and the complexity of these signals is just now being worked out. Micro RNAs help balance the need for more long-term stem cells versus new blood cells. There are many ways that these signals operate. They affect the activation of the stem cells, their movement and the number of times they divide.
Micro RNAs do not code for proteins, but rather bind to specific messenger RNAs altering their function. What makes this complex is that signals from immune cells involved in inflammation change micro RNAs. They can alter how DNA makes the RNAs. They can affect how small RNAs are stabilized to maintain their functions. They can affect how they interact with the messenger RNA. Stress responses are vital in how these small micro RNAs are utilized. If these mechanisms go awry, then drastic consequences can occur, such as leukemia and autoimmune diseases.
Previous research into micro RNAs function in immune systems was based on protein transcription factors that alter DNA function directly. However, recent research shows that regulation is much more complex. One significant method of regulation involves genes that are particularly sensitive to the question of quantity—that is genes where a small change produces a major shift in the numbers of cells.
 Another way they are important is in choices between producing two different immune cells. This is especially the case when the choice is close and small input can have a big result. These small microRNAs appear vital for positive and negative feedback in these close call situations. Seemingly small distinctions can have major impact on disease. In fact, multiple small microRNAs can work together in these circumstances either cooperatively or the opposite. There are now a large number of microRNAs found in these regulatory networks. Research is beginning to use them as treatments.
Another way they are important is in choices between producing two different immune cells. This is especially the case when the choice is close and small input can have a big result. These small microRNAs appear vital for positive and negative feedback in these close call situations. Seemingly small distinctions can have major impact on disease. In fact, multiple small microRNAs can work together in these circumstances either cooperatively or the opposite. There are now a large number of microRNAs found in these regulatory networks. Research is beginning to use them as treatments.
Specific mechanisms have been discovered. But, they mostly refer to the effects of one micro RNA even when it is known that many interact. The research is just beginning.
How do MicroRNAs Work?
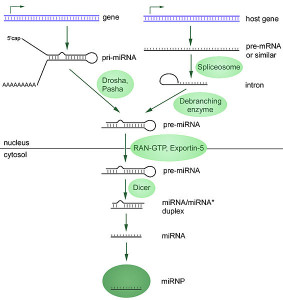
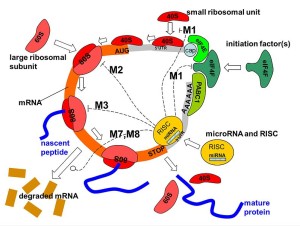
miRNAs work by altering messenger RNA and can totally change the dynamics of protein production. They are made by RNA polymerase and then cut by several enzymes that are part of the microprocessor complex. A precursor pre-miRNA is transported from the nucleus into the cytoplasm. There it is cut again into double stranded RNA by Dicer enzyme. One of these strands is taken into the large RISC complex. RISC stands for RNA-induced silencing complex. It is used by RISC to bind to messenger RNA code.
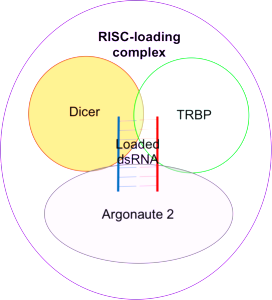
Each of these steps can be regulated in the immune processes producing more or less of particular microRNAs. Most of the time, microRNAs stop the function of the messenger RNA interfering with proteins. But, they can do the opposite and make more proteins.
“Micro RNA” is written as miRNA. Particular miRNAs are termed miR-xxx. This post will use this nomenclature for individual miRNAs. But, also sometimes use the general term micro RNA as well as miRNA.
Feedback Loops
A positive feedback loop starts with a transcription factor (A) producing the micro RNA that is important to make a protein. Another factor B inhibits A. But, when A inhibits (B), this increases A and produces more of one type of cell.
Negative feedback involves A which stimulates the pathway. B is crucial for more A. When A inhibits B, it inhibits more A.
Switches are systems which trigger two different genes. These systems involve at least three factors that can either stimulate or inhibit. An example is A regulating B and C and B regulating C. Such as system has at least 8 different scenarios. These can have many different results in immune function.
There are situations where the same micro RNA can have different effects, which also confuses outcomes.
Some processes involve alterations in narrow ranges of too many and too few proteins. Other processes affect the tipping point between the two forces. What complicates this is that the amount of RNAs determine whether they are utilized. Below a certain concentration they are inhibited and above a level they are saturated and ignored. These specific points are part of master regulation of whether a particular cell will be produced. Complicating this even further, there are competing types of RNAs that confuse and alter the critical threshold point.
Stem Cells
 Stem cells in bone marrow must produce all the necessary immune cells throughout the body. With and without stress, micro RNAs are vital in this process. One vital element is a nuclear process that binds the cap of the RNA and errors in this process create disease. These involve miR-155, let-7 and miR-21.
Stem cells in bone marrow must produce all the necessary immune cells throughout the body. With and without stress, micro RNAs are vital in this process. One vital element is a nuclear process that binds the cap of the RNA and errors in this process create disease. These involve miR-155, let-7 and miR-21.
miR-125 has two members (a and b) and maintains stem cells in normal conditions. They stop messenger RNA that produces important proteins for cell suicide. The mechanism is very complex with many pathways involving miR-99b and let-7e. In research, more miR-125a, working in a cluster, increases cells by 10 times and involves the vital factor related to cell death and reproduction, BCL-2. This can induce leukemia of both lymphocytes and myeloid cells.
 miR-125b has a different cluster of miR-99a, miR-100 and let-7c. This leads to activation of multiple very complex pathways. Two competing cytokines regulate more stem cells versus more derivative cells. These competing forces create an important switch.
miR-125b has a different cluster of miR-99a, miR-100 and let-7c. This leads to activation of multiple very complex pathways. Two competing cytokines regulate more stem cells versus more derivative cells. These competing forces create an important switch.
miR-29a is important in bone marrow stem cells. It pushes in the direction of myeloid cells and is related to cell suicide as well.
miR-22 and miR-124 are involved in tags that are placed on DNA. These impair the start of the reproduction cell cycle in the stem cell.
miR-126 regulates the number of stem cells and can stimulate more cells without appropriate regulation of producing too many cells. Normally, a process called exhaustion will stop processes that have gone awry. This leads to stem cells causing leukemia.
Let-7 is a family of micro RNAs that are modified by tags. Complex feedback loops both positive and negative regulate how fetal cells become adult versions.
miR-193 and miR-132 are stimulated by signals from immune cells. One inhibits particular cytokines related to producing blood cells. The other targets pathways that work for and against certain cytokines. Two other micro RNAs, miR-221 and miR-222 interact in these cycles in complex ways.
miR-212 and miR-132 helps the aging stem cell work against accumulation of reactive oxygen. These are part of a cluster that is increased in aging cells. These two buffer the levels of essential proteins to combat effects of aging.
Immune Responses
Vital cells in the myeloid line include macrophages that are highly regulated by micro RNA processes. miR-155 and miR-146a are stimulated by other immune cells in both dendritic cells and macrophages. Pattern recognition receptors and many cytokines trigger NF-κB and activator protein 1 (AP-1). These inhibit miR-155 and miR-125b and stimulate let-7, which triggers other pattern receptors.
The result of this stimulation is rapid increase and then stopping, creating a pulse of activity. Antagonistic forces increase inflammation and more macrophages.
Granulocytes
 Granulocytes are a category of white blood cells that have different compartments of molecules that are seen by microscope. The most prominent is the leukocyte, but others include eosinphils, basophils and mast cells (with histamine granules).
Granulocytes are a category of white blood cells that have different compartments of molecules that are seen by microscope. The most prominent is the leukocyte, but others include eosinphils, basophils and mast cells (with histamine granules).
miR-223 dramatically affects granulocyte development. This microRNA is involved in multiple ways including a switch. One result is more of all granulocytes. Others are vital as well, miR-21, miR-130a and miR-196b.
Natural Killer Lymphocytes
T cells can produce large numbers of natural killer cells to attack microbes and cancer. miR-181 is vital for early production. These processes stimulate many cytokines including IL-4 and IL-17.
Research into micro RNA effects in platelets and red blood cells is just beginning and little is known. miR-150 increases megakaryocytes and miR-144 and miR-451 increase red blood cells impacting on several genes. miR-221 and miR-222 are increased in mast cells.
T and B Lymphocytes
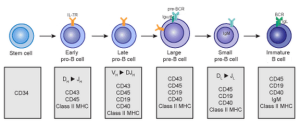
There is a large complex literature of micro RNA effects related to lymphocytes. B-lymphocytes have multiple levels of stem cells—the pre-pro-B cells, pro-B cells and then pre-B cells. Different genetics networks produce each of these. Vital genes to produce immunoglobulin start in the pre-B cells. Newly minted B cells produce the critical receptors. Micro RNA effects are complex. At first miR-181 was thought to influence the entire process. Many different micro RNAs are being found that affect production of this sequence of cells.
miR-150 can cause cell death in pro-B cells. This microRNA inhibits a factor that can lead to cancer. This micro RNA and miR-132 have vital antagonistic roles that buffer the switch for this transition.
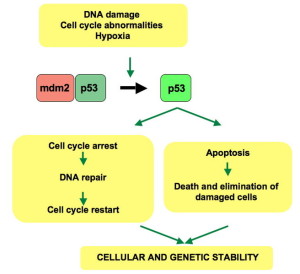 The vital p53 stress pathway (cell death and reproduction) stimulates miR34a but inhibits pro-B to leading to pre-B through a gene that is known to cause cancer (oncogene). Another cluster miR-212 and miR-132 inhibit the previous step of pre-pro B to pro-B. Both of these mechanisms create switches. To continue concentrations must go above thresholds. A different mechanism has miR-17-92 clusters that stop cell suicide pathways and make the transition go through.
The vital p53 stress pathway (cell death and reproduction) stimulates miR34a but inhibits pro-B to leading to pre-B through a gene that is known to cause cancer (oncogene). Another cluster miR-212 and miR-132 inhibit the previous step of pre-pro B to pro-B. Both of these mechanisms create switches. To continue concentrations must go above thresholds. A different mechanism has miR-17-92 clusters that stop cell suicide pathways and make the transition go through.
A latter step makes plasma cells from B cells by inhibiting miR-21, which can cause cancer. This transition needs miR-155 and miR-148a, which have multiple complex pathways and feedback loops.
Fetus B cell development veers towards a subclass called B1 that can not produce memory cells. Let-7 drives the transition to adult B2 cells that do have memory.
B Cell Immune Function
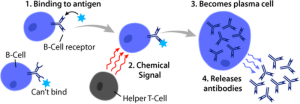
Micro RNAs are vital for the B cells antibody response. miR-142 is necessary for antibody production. miR-146a is necessary not to produce antibodies against human cells. miR-155 was the first noted to be vital in the special process B cells use to hone antibodies to make them more accurate. This process somatic hypermutation is also regulated by actions of T cells (see post). These create positive feedback. Negative feedback is created by miR-181b. Another miR-210 also causes negative feedback with defective antibodies. miR-217 interferes with DNA repair and helps cause lymphoma (cancer of lymphocytes).
T Cells
T cells borne in the bone marrow travel to the thymus to develop and be trained. T cells pass through training to produce vital receptors and go through a series of stages where they must pass tests of producing these receptors. Many different micro RNAs have recently been found that are vital in this training process to help get over some of the check points in T cell training. miR-181a serves as a switch combined with several complex pathways. It can impair several checkpoints.
One example occurs in the production of T helper cell 17, which is stimulated by several cytokines. A previous post showed how specific helper T cells are stimulated by food particles in order to avoid autoimmune reactions to that food (see post.) One of the main receptors works in pathways with miR-155. Another interacting pathways is triggered by miR-21 and an opposite one by miR-301, miR-23 and miR-326. All of these are counteracted by miR-20b. Smaller roles in this vital stimulation of T cells are through miR-212 and miR-132.
When T cells are triggered by material from an invading microbe, the complex process of activation occurs. This includes several other cells participating in the final decision. Once activation occurs, then the T cell becomes a powerhouse of trouble, making armies of killer cells. Very recent research shows that this process involves the interaction of multiple micro RNAs.
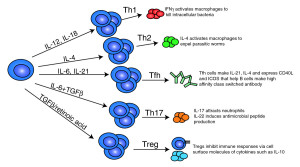 miR-181a affects the receptors threshold for action and encourages production of new cells. A micro RNA cluster miR-17-92 has two components in this process, miR-17 and miR-20a.
miR-181a affects the receptors threshold for action and encourages production of new cells. A micro RNA cluster miR-17-92 has two components in this process, miR-17 and miR-20a.
Members of this cluster independently affect multiple pathways that ultimately have the same result. miR-19b and miCR17 both help produce special T helper cells. miR-182 gives positive feedback and pushes the process further. miR-24 also helps and miR-27 inhibits through several cytokines.
Two micro RNAs produce inflammation, miR-146a and miR-155, but in antagonistic ways. They both affect specific cytokines in different ways.
Blood Cancers and Autoimmune Diseases
 Very complex interactions of micro RNAs are involved in multiple sclerosis, lupus, inflammatory bowel disease and blood cancers. Micro RNAs that stimulate cancers are now called oncomirs, similar to the term oncogene for a gene causing cancer. Many other micro RNAs suppress cancer through similar pathways.
Very complex interactions of micro RNAs are involved in multiple sclerosis, lupus, inflammatory bowel disease and blood cancers. Micro RNAs that stimulate cancers are now called oncomirs, similar to the term oncogene for a gene causing cancer. Many other micro RNAs suppress cancer through similar pathways.
miR-125b is an oncomir causing both cancers of myeloid (white blood cells) and lymphocytes. They interact with pathways in stem cells that make new cells. miR-28 suppresses lymph cancers. When miR-28 is inhibited, cancers are formed. miR-21 stimulates B lymphocyte lymphoma and inhibition of it kills the cancer. There are many other complex pathways related to micro RNA clusters that have effects on cancer.
Chronic inflammation can cause cancer. miR-155 interferes with inflammation regulation causing leukemias.
Multiple micro RNAs appear to be involved in autoimmune diseases but the mechanisms are not yet clear. Multiple different pathways involve miR-20b, miR-21, miR-212, miR-132, miR-301, miR-326 and the cluster miR-17-92. One mechanism includes miR-146a affecting B and T cell activity and miR-155 leading to arthritis against collagen molecules.
Vast Complexity of Immune Micro RNA Signals
Only 2% of DNA codes for proteins and fifty percent are jumping genes. This leaves the possibility of 48 percent of DNA making large and small non coding RNAs. This is thirty times greater than the DNA that codes for proteins. There is no way to know how much of these are important regulatory particles. But, ENCODE (Encyclopedia of genetic elements) shows it is possibly 20 percent, which is ten times greater than coding DNA.
In any case, it is clear that a vast number of different large and small RNAs are vital for all aspects of physiology. Micro RNAs with the very specific RISC mechanism for inhibition of messenger RNAs seems particularly vital. Along with all of the other regulatory signals (neurotransmitters, cytokines, proteins, lipids, sugars, peptides), regulation is vastly complex.
This complexity raises the question of where the direction is for all of this. It is absurd to think that this is the result of random processes.