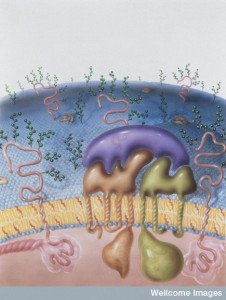
 A previous post discussed the great complexity of cell membranes and the varied lipids that are manufactured, tagged and transported for many different membranes—vesicles, signaling, and cellular compartment structures. Cholesterol is one of the key lipids with many functions. Another special molecule—a lipoprotein, combining lipids with protein—transports cholesterol and has been widely discussed in the media as an important risk factor for heart disease. Low-density lipoproteins (LDL) not only transport cholesterol in the blood, but also have a wide range of other critical functions in the brain.
A previous post discussed the great complexity of cell membranes and the varied lipids that are manufactured, tagged and transported for many different membranes—vesicles, signaling, and cellular compartment structures. Cholesterol is one of the key lipids with many functions. Another special molecule—a lipoprotein, combining lipids with protein—transports cholesterol and has been widely discussed in the media as an important risk factor for heart disease. Low-density lipoproteins (LDL) not only transport cholesterol in the blood, but also have a wide range of other critical functions in the brain.
Lipoproteins are manufactured, modified and tagged for transport in the endoplasmic reticulum (ER) and the Golgi apparatus in a very elaborate process. While protein regulation is extremely complicated, the addition of lipids and sugars to proteins creates complexity orders of magnitude greater. Lipoproteins are just one of thousands of large molecules combining proteins with lipids and sugars. LDL is one type that has hundreds of varieties. This post will describe the unique and complex functions of the very versatile lipoproteins in healthy brains and Alzheimers disease. This is why such a memory care facility exists to help those with Alzheimer’s that are struggling with their memory.
Lipoproteins Are Complex Molecules Built in the ER and Golgi
 In the endoplasmic reticulum (ER) and Golgi, lipids are bound to proteins. This combination allows lipids to move in water outside and inside the cell. The lipoprotein emulsifies the fats—equivalent to shaking an oil and water salad dressing, where small pieces of fat become dissolved in the water.
In the endoplasmic reticulum (ER) and Golgi, lipids are bound to proteins. This combination allows lipids to move in water outside and inside the cell. The lipoprotein emulsifies the fats—equivalent to shaking an oil and water salad dressing, where small pieces of fat become dissolved in the water.
Lipoproteins have a very wide range of functions including building cellular compartments, serving as vehicles for transporting molecules, helping neurons migrate by adhering to other cells, serving as antigens triggering antibodies, as well as participating in enzymatic reactions. The most famous lipoproteins are HDL and LDL (high density and low density lipoproteins) that carry fats in the blood stream and are measured as risk factors for cardiac disease. Many other lipoproteins are large complex molecules that lie in the membranes of mitochondria and there are many lipoproteins involved in the fight against microbes.
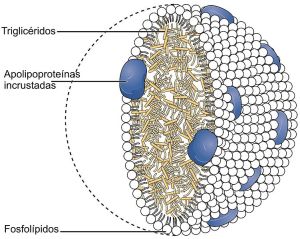
There are five basic classes of lipoproteins with LDL being the second smallest—the others are (largest to smallest) chylomicrons, very low-density lipoproteins (VLDL), intermediate density lipoprotein, and HDL. The largest lipoproteins, chylomicrons, carry food in the blood. In fact, there are thousands of different LDL and HDL types and only some are related to cardiac risk factors. The LDL of bad cholesterol transports fats to arteries, attracts macrophages and increases clogged arteries. HDL, good cholesterol, can remove the fat.
Very low-density lipoproteins (VLDL) are made of apolipoproteins (involved with Alzheimer’s – discussed below) and allow fats and cholesterol to move in the blood. They are turned into LDL. Apolipoproteins are components of lipoproteins that add
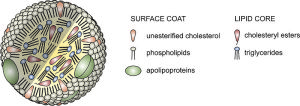
structure to the large molecule. They are, also, factors that help receptors serve as enzymes. ApoE, the most famous apolipoprotein, is able to help in the transport of cholesterol and other lipids in the blood because it is highly attractive to many receptors on cellular surfaces.
Low density lipoproteins (LDL), despite a bad reputation in the popular press, actually have very important roles in the development of adult brains, development of synapses, transportation of many different cargos and in signaling cascades that stimulate genetic networks. They have been found to be important in Alzheimer’s disease and other brain diseases.
LDL Receptors
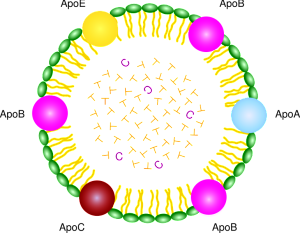
There are multiple critical molecules that are part of the system related to the LDL receptors. These receptors sit in the membrane and include a wide variety of molecules and structures that are specific for different types of signaling. The many different molecules that stimulate these receptors are related to the wide range of functions.
LDL receptors interact with a very large amount of critical pathways and enzymes—sonic hedgehog, Wnt, Reelin, apolipoproteins ApoE and ApoB, protease inhibitors, chaperones for protein folding, immune receptors, and molecules that carry vitamins.
These many different receptors play a vital role in the fetal brain, adult brain, neuroplasticity and brain cell’s migration. They are the receptors for the most well known risk factor for Alzheimer disease, ApoE4.
Cholesterol and Lipoprotein Receptors
 LDL receptors are most widely known for transport of cholesterol in the blood. Cholesterol is needed for hormones and membranes and is transported by lipoproteins to the cells. The receptors bind to lipoproteins that carry cholesterol and triglycerides and take them into the cell through complex process.
LDL receptors are most widely known for transport of cholesterol in the blood. Cholesterol is needed for hormones and membranes and is transported by lipoproteins to the cells. The receptors bind to lipoproteins that carry cholesterol and triglycerides and take them into the cell through complex process.
A similar process occurs in the brain. Special lipoproteins are manufactured in the brain since they can’t cross the blood brain barrier. Glia make cholesterol, which is necessary for creating and maintaining synapses. Astrocytes use a version of HDL, containing the molecule ApoE, to transport phospholipids and cholesterol among the various brain cells. ApoE binds to a large number of different receptors in brain cells. These receptors create a large number of vesicles inside the cell.
Lipoproteins in the Fetal Brain
Multiple unique receptors are active in the fetal brain. A variety of diseases are caused by impaired lipoprotein receptors. Reelin, a very large glycoprotein secreted into the extra cellular matrix, is a critical molecule in the developing brain. Reelin, necessary for brain development and later adult function, uses the Apoe receptors.
Lipoproteins in Neuroplasticity
 Both LDL and VLDL lipoprotein receptors exist in microglia, astrocytes and the large postsynaptic density of the postsynaptic neuron. ApoE receptors are widely distributed in the brain sending material into the postsynaptic density at synapses. ApoE receptors work with the glyocoprotein Reelin and are critical to create long term potentiation of neuroplasticity.
Both LDL and VLDL lipoprotein receptors exist in microglia, astrocytes and the large postsynaptic density of the postsynaptic neuron. ApoE receptors are widely distributed in the brain sending material into the postsynaptic density at synapses. ApoE receptors work with the glyocoprotein Reelin and are critical to create long term potentiation of neuroplasticity.
Reelin binds to clusters of complex receptors triggering many different cascades inside the cells. These processes increase calcium flux and increase AMPA receptors in NMDA neurons. See posts on neuroplasticity for details of these mechanisms. In fact, Reelin injections into the brain ventricles increase memory in mice.
There are a many different Reelin receptors, including LDLs, which affect brain growth and function. As well as affecting synapses, Reelin triggers vesicles for neurotransmitters from the presynaptic neuron. They work with integrin and affect vesicle fusion and release.
Lipoproteins in the Peripheral Nervous System
 Lipoprotein receptors are critical, also, for the neuro muscular junction, which is a cholinergic synapase with muscle cells. These receptors trigger the pattern of the future muscle as well the creation of clusters of receptors for future muscle function. When the connection is built, lipoprotein receptors signal backward from the muscle endplate to the neuron to stimulate more receptors and neurotransmitter vesicles.
Lipoprotein receptors are critical, also, for the neuro muscular junction, which is a cholinergic synapase with muscle cells. These receptors trigger the pattern of the future muscle as well the creation of clusters of receptors for future muscle function. When the connection is built, lipoprotein receptors signal backward from the muscle endplate to the neuron to stimulate more receptors and neurotransmitter vesicles.
Amyloid precursor protein, APP, is involved in the muscle junction as well. It works on both pre and postsynaptic aspects of the synapse.
Lipoproteins In Alzheimer’s
A mutation in the amyloid precursor protein, APP, or the enzymes that cut it, can lead to Alzheimer’s disease. These mutations exist only in the early onset version that is a small percentage of the total patients. It leads to a buildup of the toxic shorter version of the amyloid-? (see the post on Inflammation and Dementia for more details).
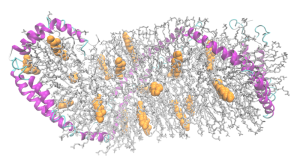 The major risk factor for the late onset Alzheimer’s, which is most of the cases, involves a specific version of apolipoprotein E, (?4). There are, in fact, three different versions, ?2, ?3 and ?4. ApoE3 is the most frequent in the population. ApoE4 is in approximately 15% of the population and greatly increases the risk of Alzheimer’s. With ApoE2 there is less Alzheimer’s than the other types. These different versions create varied protein shapes, with altered receptor binding and effects on creating vesicles.
The major risk factor for the late onset Alzheimer’s, which is most of the cases, involves a specific version of apolipoprotein E, (?4). There are, in fact, three different versions, ?2, ?3 and ?4. ApoE3 is the most frequent in the population. ApoE4 is in approximately 15% of the population and greatly increases the risk of Alzheimer’s. With ApoE2 there is less Alzheimer’s than the other types. These different versions create varied protein shapes, with altered receptor binding and effects on creating vesicles.
It has been very hard to pin down exactly how this difference occurs. Because of the great complexity of studying the creation of vesicles, the signaling of the lipoprotein receptors and defective synapses, it is possible that mechanisms could be related to hundreds of different factors.
Vesicles Pathways and Lipoproteins
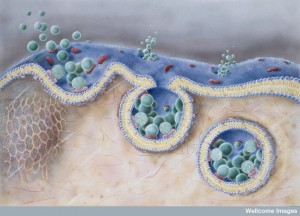 A previous post described the complex cellular pathways (pipeline) where lipids are transported throughout the cell. Compartments in these pathways are called endosomes. Endosomes exists all along the pipeline throughout the cell.
A previous post described the complex cellular pathways (pipeline) where lipids are transported throughout the cell. Compartments in these pathways are called endosomes. Endosomes exists all along the pipeline throughout the cell.
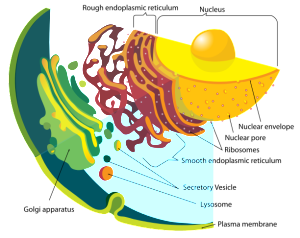
Proteins are manufactured just outside of the nucleus in the rough endoplasmic reticulum (called rough because of the many ribosomes making proteins). Lipids are made in the contiguous smooth ER. Proteins and lipids are put together in the ER to make lipoproteins and then they are modified in the Golgi (see post). The Golgi tags them for special transmit to the many places where they are used. The transit goes from ER and Golgi to mitochondria, to all organelles, to the outer membrane and to the many vesicle structures, such as lysososomes.
In the transit system of endosomes, molecules can go from the cell outer membrane to lysosomes to be destroyed, or be recycled into other forms and returned to that membrane or other organelles. They can be recycled through the Golgi or back to the Golgi.
Critical APP Altered in the Lipid Pipeline
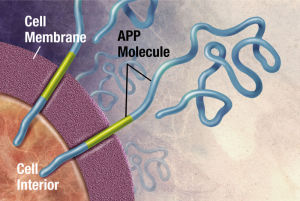 App, the molecule that is the precursor to amyloid-?, is cut by the enzymes beta secretase and gamma secretase creating the amyloid-? molecules. These two enzymes have a variety of functions in the synapse. One is related to pruning synapses, stopping new brain cell creation and increasing cell death. The other is related to protection of synapses.
App, the molecule that is the precursor to amyloid-?, is cut by the enzymes beta secretase and gamma secretase creating the amyloid-? molecules. These two enzymes have a variety of functions in the synapse. One is related to pruning synapses, stopping new brain cell creation and increasing cell death. The other is related to protection of synapses.
The cutting process makes 2 types of amyloid-? with different numbers of amino acids—either 40 amino acids or 42. The 42 variety creates the plaques of Alzheimer’s. The mutation mentioned above, also, increases the 42 version.
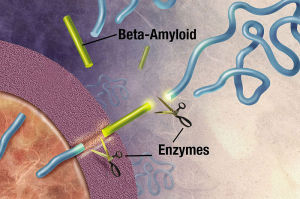 The APP and the secretase are usually in different compartments of the pipeline, but in Alzheimer’s they share the same compartment and produce more of the 42 variety.
The APP and the secretase are usually in different compartments of the pipeline, but in Alzheimer’s they share the same compartment and produce more of the 42 variety.
One of the first problems in Alzheimer’s involves the recycling of the lipid pathways. Some increase to 30 times the size of normal compartments.
There are many different lipoprotein receptors that affect the cycling of the lipids in the pipeline and secretory pathways. There are many different mechanisms that seem to be involved in the creation of abnormal compartments and trafficking and the production of amyloid-?. These defects appear between the ER and the Golgi.
Lipid Rafts in Membranes, Lipoproteins and Alzheimer’s
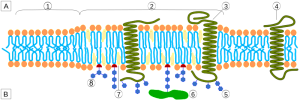 In the membrane, there are special structures that organize a large amount of activity, including many different types of receptors and the connections to the cascades inside the cell. These structures, called lipid rafts, are made of lipids, proteins attached to lipids (lipoproteins), and proteins attached to both sugars (glycoproteins) and lipids (glycolipoproteins). The rafts, also, change the characteristics of the membrane in terms of flexibility; create an organization for large signaling molecules lying in the membrane; and regulate transport of receptors into the membrane and secretion of neurotransmitters.
In the membrane, there are special structures that organize a large amount of activity, including many different types of receptors and the connections to the cascades inside the cell. These structures, called lipid rafts, are made of lipids, proteins attached to lipids (lipoproteins), and proteins attached to both sugars (glycoproteins) and lipids (glycolipoproteins). The rafts, also, change the characteristics of the membrane in terms of flexibility; create an organization for large signaling molecules lying in the membrane; and regulate transport of receptors into the membrane and secretion of neurotransmitters.
Although the rafts are highly organized and very compact, they float in the membrane. They exist in both the outer cellular membrane and in the membranes of organelles, such as Golgi and lysosomes.
The 42 version of amyloid-? affects the lipid rafts. The ApoE2 alters the lipid rafts, providing an increased amount of APP and an increased amount of amyloid-? of the bad variety
Amyloid-? and Synapses
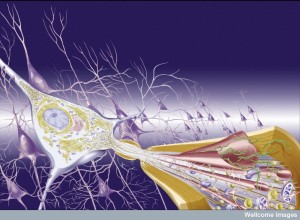 It is not clear exactly how amyloid-? affects synapses. One study showed that a low level of normal amyloid-? decreases memory. Strangely, more use of the synapse increases the amount of amyloid-? in the extracellular space and decreases the amount inside the cell by two separate mechanisms.
It is not clear exactly how amyloid-? affects synapses. One study showed that a low level of normal amyloid-? decreases memory. Strangely, more use of the synapse increases the amount of amyloid-? in the extracellular space and decreases the amount inside the cell by two separate mechanisms.
When amyloid-? increases in the extracellular space it decreases neuroplasticity. This is related to the subtle process of synapse regulation where it is decreased when the amount of signaling is too high. It is a form of scaling in relation to other neuronal signals in the circuit. The synapses with less activity then become more ready for stimulation.
When amyloid-? combines to form oligomers (the beginning of clumps and plaques), LTP is decreased through very complex mechanisms. This is reversed through increased Reelin with ApoE receptors of various types. The receptors involved are part of a recycling process where by new receptors are brought to the membrane in vesicles. The transport process of this recycling is impaired with ApoE2.
Reelin in Alzheimer’s
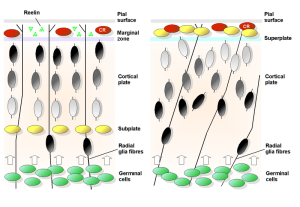 Reein levels are reduced in critical brain regions in Alzheimer’s. Some regions try to compensate with increased production of amyloid-?.
Reein levels are reduced in critical brain regions in Alzheimer’s. Some regions try to compensate with increased production of amyloid-?.
Another cascade from Reelin leads to increase in tau hyperphosphorylation, the other key sign of Alzheimer’s, called neurofibrillary tangles.
Normally, tau 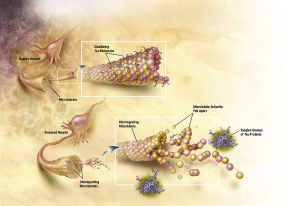 holds together microtubules that provide the structure and transport of the neuron. When tau has too many phosphates added, the microtubules break apart causing the neurofibrillary tangles (NFT). Also, these NFTs travel from the axon to the cell body where they destroy the functions of the cell. There seems to be many different ways that Reelin can cause these changes. The research is complicated by different mechanisms in different animal studies. With many different effects of reelin it has not been possible to find the exact mechanisms yet.
holds together microtubules that provide the structure and transport of the neuron. When tau has too many phosphates added, the microtubules break apart causing the neurofibrillary tangles (NFT). Also, these NFTs travel from the axon to the cell body where they destroy the functions of the cell. There seems to be many different ways that Reelin can cause these changes. The research is complicated by different mechanisms in different animal studies. With many different effects of reelin it has not been possible to find the exact mechanisms yet.
Clearance of ApoE and Amyloid-? in Alzheimer’s
 The ways that misfolded proteins and potentially toxic byproducts like amyloid-? are cleared from the brain are critical to brain function. Please see the post on Five Secrets of Brain Health for the dramatic finding of how sleep triggers increased cleaning in the brain by expanding the extracellular space and the fluid flow between blood vessels and the cerebrospinal fluid.
The ways that misfolded proteins and potentially toxic byproducts like amyloid-? are cleared from the brain are critical to brain function. Please see the post on Five Secrets of Brain Health for the dramatic finding of how sleep triggers increased cleaning in the brain by expanding the extracellular space and the fluid flow between blood vessels and the cerebrospinal fluid.
There are, however, many clearance mechanisms. One is transport across the blood brain barrier. Another is by destruction by lysosomes (vesicles that cut up molecules). A third is by special enzymes, called proteases, that cut up the amyloid-?.
In the late onset Alzheimer’s, defective clearance is possibly the most important factor. The amyloid-?42 is cleared more slowly, to begin with. This leads to less amyloid-?40 and more amyloid-?42. ApoE4, also, slows the clearance. There are multiple different ApoE receptors involved in this process.
 Normally, in the blood amyloid-? is rapidly cut up. In Alzheimer’s, the lipoprotein receptors that rapidly send amyloid-? across the blood brain barrier become defective. When the blood brain barrier is damaged in some types of Alzheimer’s, there is no regulation left and amyloid-? can leak back into the brain. ApoE4 is, somehow, important in the regulation of the blood brain barrier functions.
Normally, in the blood amyloid-? is rapidly cut up. In Alzheimer’s, the lipoprotein receptors that rapidly send amyloid-? across the blood brain barrier become defective. When the blood brain barrier is damaged in some types of Alzheimer’s, there is no regulation left and amyloid-? can leak back into the brain. ApoE4 is, somehow, important in the regulation of the blood brain barrier functions.
ApoE3 increases the transport of amyloid-? to lysosomes and special enzymes that cut it. ApoE4, also, has defective effects on these clearance mechanisms. A special enzyme is released from microglia and astrocytes to clear amyloid-? and these are, also, less with ApoE4.
More Complexity in Lipoprotein Function and Alzheimer’s
Special processes transport cholesterol to the ApoE complex. This process of adding more cholesterol appears to help with the clearance of amyloid-?. ApoE attracts and binds amyloid-? much more strongly when it has more cholesterol.
 There are other mechanisms, as well, using lipoproteins. For example, oligodendrocytes secrete galactocerebrosides that is a major component of myelin. These molecules make a complex with suflides. They, also, increase the binding of loose amyloid-?. In Alzheimer’s there is a decreased amount of sulfide, and therefore the galactocerebrosides mechanism. When there is less sulfide there is less function of the lipid transport. This change is specific to Alzheimer’s and is meditated by lipoprotein receptors.
There are other mechanisms, as well, using lipoproteins. For example, oligodendrocytes secrete galactocerebrosides that is a major component of myelin. These molecules make a complex with suflides. They, also, increase the binding of loose amyloid-?. In Alzheimer’s there is a decreased amount of sulfide, and therefore the galactocerebrosides mechanism. When there is less sulfide there is less function of the lipid transport. This change is specific to Alzheimer’s and is meditated by lipoprotein receptors.
A totally unrelated effect of ApoE4 is hyperactivity in brain regions that produces more amyloid-?. It is not clear which is cause and which is effect—more amyloid-? or more hyperactivity.
Vascular Lipoprotein Receptors
Amyloid can be deposited in small blood vessels of the brain. This defect can cause blood to seep out of the vessels, producing hemorrhages with brain damage. This process, also, occurs in Alzheimer’s patients, which causes additional brain damage. This is one way where most patients with Alzheimer’s disease, also, have some form of vascular dementia. ApoE2 is protective for Alzheimer’s but increases these hemorrhages.
Perhaps Lipid Transport and Pipeline Are Most Important Factors in Alzheimer’s
 What is, perhaps, most significant is that the lipid regulatory pathways and the pipeline (described in previous post, Amazing Complexity in the Membrane) are the major connections to all of these processes related to Alzheimers.
What is, perhaps, most significant is that the lipid regulatory pathways and the pipeline (described in previous post, Amazing Complexity in the Membrane) are the major connections to all of these processes related to Alzheimers.
In Alzheimer’s ApoE4 abnormally places APP and critical enzymes in the same compartments. It stops transport of ApoE4 between neurons causing buildup. It, also, lowers Reelin receptors, blocking Reelin’s protective function from amyloid-?.
Therefore, it is possible that approaches to lipid transport could be vital in curing Alzheimer’s. Drugs that affect the transport of lipids might be able to help. While statins have not clearly worked, others are being researched.
Microbes and LDL
While microbes have their own versions of lipoproteins, human LDL has a unique effect on microbes. Microbes use a signal to create group activity. LDL interferes with the group signal (quorum sensing) of dangerous Staph aureus. The apolipoprotein binds to a genetic factor in the microbe stopping the communication.
Versatile Lipoproteins in Healthy Brains and Alzheimers
While the manufacture and regulation of proteins is extremely complicated, an entirely new level of complexity is being discovered with proteins attached to thousands of types of lipids and sugars. These lipids and sugars make an almost infinite variety of shapes and structures. The manufacture, modification, tagging and transport of these combination molecules uses extremely elaborate mechanisms for very specific varied structures and functions. Just one of these molecules, LDL, has many varied critical functions and is involved in many different mechanisms related to Alzheimer’s disease.
 Messenger RNA comes through the holes in the nucleus membrane (nuclear pores) into the endoplasmic reticulum, made of extremely complex membranes with elaborate machinery to manufacture proteins. Once manufactured, proteins travel to the outer ER and are built into lipo-proteins and glyco-proteins of all kinds. These are sent to the Golgi, where they are modified, tagged and transported to specific locations along the pipeline throughout the cell. They, also, return to the Golgi after being used to either be recycled or destroyed.
Messenger RNA comes through the holes in the nucleus membrane (nuclear pores) into the endoplasmic reticulum, made of extremely complex membranes with elaborate machinery to manufacture proteins. Once manufactured, proteins travel to the outer ER and are built into lipo-proteins and glyco-proteins of all kinds. These are sent to the Golgi, where they are modified, tagged and transported to specific locations along the pipeline throughout the cell. They, also, return to the Golgi after being used to either be recycled or destroyed.
Molecules with lipids and sugars attached to proteins fulfill all kinds of vital functions throughout the cell. Proteins, and their regulation, are extremely complex. But, adding thousands of different shaped lipids and sugars to proteins magnifies the complexity by many orders of magnitude?
Where is the direction for all of this construction and modification in the ER and Golgi systems? Certainly, it isn’t in the nucleus. The vast cavernous cisternae of the ER and the Golgi appear to have their own sources of direction.