 White blood cells (also named leukocytes) are called into action with microbe attacks and tissue damage of all kinds. They use very specific modes of travel to the inflammation site, spurred by the signals of capillaries, tissue cells, and other immune cells (these have been described in previous posts). Leukocytes travel through rough terrain, even without oxygen to the inflamed location based on these conversations. Recently it was found that signals tell leukocytes when the job is done, and they are often given instructions that send the leukocytes travelling to other places in the body for other fights.
White blood cells (also named leukocytes) are called into action with microbe attacks and tissue damage of all kinds. They use very specific modes of travel to the inflammation site, spurred by the signals of capillaries, tissue cells, and other immune cells (these have been described in previous posts). Leukocytes travel through rough terrain, even without oxygen to the inflamed location based on these conversations. Recently it was found that signals tell leukocytes when the job is done, and they are often given instructions that send the leukocytes travelling to other places in the body for other fights.
Only very recently, however have the signals been found in chronic inflammation situations, where white blood stay working beyond what is helpful. Not surprisingly, it is found that they have elaborate communication with tissues and immune cells even over the long haul. This post focuses on newly discovered signaling related to their role in chronic inflammation. A brief summary of the previous information about leukocyte travel is first.
Summary of Previous Information
 White blood cells are able to find sites of infection and trauma through elaborate signaling. They travel in blood vessels and into the tissue, despite many obstacles. All the dramatic types of travel are completely controlled by signals from many participating cells in the blood vessels, the tissue, and the immune and nervous systems.
White blood cells are able to find sites of infection and trauma through elaborate signaling. They travel in blood vessels and into the tissue, despite many obstacles. All the dramatic types of travel are completely controlled by signals from many participating cells in the blood vessels, the tissue, and the immune and nervous systems.
Leukocytes were thought to be only short lived using programmed suicide after their tasks. Now, more complex behavior has been observed. Leukocytes send very elaborate signals from the site and even return to the blood for more work in other places.
They respond to chemical gradients and cytokine signaling with cells and extracellular matrix. Migrating cells are able to alter their shape and modes of transport as needed. Leukocytes use back and forth communication with many other cells to analyze the best way to traverse specific terrain, as well as the specific response to the target problem. They move between lining cells with tight junction and through the dense basement membrane (which is altered by conversations to allow entry) into the extracellular space. Once in the tissue they meet very different factors including other chemical gradients that attract and repel them.
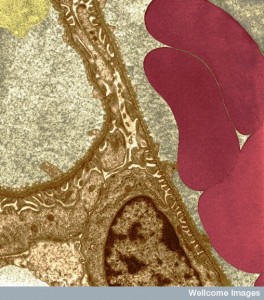 Individual cells have multiple different kinds of movement for different three-dimensional environments, provided by operation of specific actin-myosin motors, dynamic scaffolding structures, and transit tubules.
Individual cells have multiple different kinds of movement for different three-dimensional environments, provided by operation of specific actin-myosin motors, dynamic scaffolding structures, and transit tubules.
Once at the site, options include eating debris and microbes through phagocytosis.
They use toxic chemicals that are stored in their prominent granules. They produce molecules called reactive oxygen species (ROS) which can increase inflammation response and kill microbes. ROS are also used as regulatory signals. They use many other cytokine signals and produce many different kinds of enzymes that cut proteins, called proteases. The products of the cut can be other signals.
At the site they can exhibit very complex coordinated clustering formations that have been likened to insect swarming behavior and hence the term neutrophil swarming.
Functions at the site include:
- More than 30 neutrophil receptors include those that recruit them and allow them to attach to structures.
- Granules hold more than 300 different proteins that are used at different times. There are many types of granules that are released at different times. With signals from capillaries they involve attachment, and later enzymes and toxins to kill microbes. These latter also signal to other cells.
- Reactive oxygen products such as hydrogen peroxide (H2O2) and

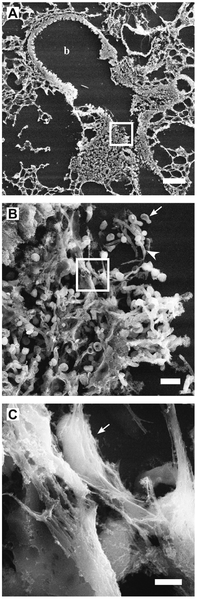
NETs from Pixie~commonswiki hypochlorous acid (HCLO) are used to kill microbes and dissolve damaged material. They are also signals when they alter proteins into other signals.
- Programmed cell death is used as part of the plan to tamp down inflammation.
- Autophagy is the process of eating debris and recycling material in cells.
- They produce an unusual structure called neutrophil extracellular traps or NETs made up of a scaffold produced from DNA, histones, and other proteins. NETs help the enzymes attack tissue and microbes by holding onto microbes. They are also signals to other cells such as DCs.
- Micro particles are proteins that are combined into a package used for many purposes.
- Genetic networks have several programs that are used in various situations.
- MicroRNA are used as various signals.
When injuries are repaired, inflammation is tamped down. Leukocytes are removed from the site in multiple ways. One is cell death either programmed suicide or killed by other cells and macrophages eat this debris. Leukocytes can be directed to move backwards out of the inflammation site using signals to cross back into the blood from the tissue.
White Blood Cell Activities

Three abnormal states in tissues produce either new abnormal types of cells, destruction of existing normal cells, and many kinds of inflammation.
Inflammation is the most varied and the most common. It responds to the wide range of circumstances including a vast number of different attacking microbes, many different kinds of damage from toxins, radiation, as well as blunt trauma. It is the vital factor in healing of all types. Too much inflammation becomes a chronic source of damage and because of this many types of immune cells attempt to tamp down the activity when the work is done.
Chronic inflammation is different in multiple ways. Cancer has been called the “wound that doesn’t heal” because in the milieu of cancer, genetic pathways for repair of DNA activity is damaged and many more mutations can occur. These increased mutations help cancers and they interact with alterations in local cells where they switch allegiance and become supportive of the cancer rather than the normal tissue.
White blood cells are vital in all of these processes. But, only now has their role in chronic inflammation been found. This post will focus only on this new material and not the more widely known vital contribution of leukocytes in acute inflammation responses (see previous posts).
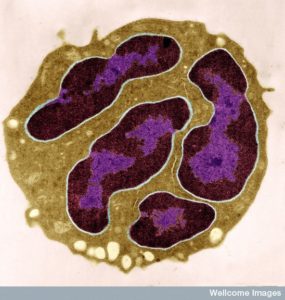
Neutrophils are the Most Common White Blood Cell
 There are multiple types of leukocytes and the most common are neutrophils. They have a short life. But, not as short as previously thought. For example, it was thought that they die after their jaunt to a particular site. It was recently shown that many live on and through conversations are directed to a second site. Still, 10 with 11 zeros are produced every day and many die quickly.
There are multiple types of leukocytes and the most common are neutrophils. They have a short life. But, not as short as previously thought. For example, it was thought that they die after their jaunt to a particular site. It was recently shown that many live on and through conversations are directed to a second site. Still, 10 with 11 zeros are produced every day and many die quickly.
Specific signals trigger more production of neutrophils such as the cytokine granulocyte colony stimulating factor (G-CSF). Neutrophils are called granulocytes because of sacs filled with granules made of proteins. It is one of the three major functions of neutrophils —to eat cells and debris (as phagocytes), to use vesicles filled with proteins to destroy and alter surroundings (granulocytes), and to create a special type of structure called Neutrophil extracellular traps (NETs), which are networks of fibers made of DNA that is secreted from the cell. NETs are used to grab onto microbes.
 G-CSF triggers genetic networks in stem cells, and to help send them into the blood. The great majority of these leukocytes are stored in the bone marrow. It inhibits receptors that hold them in the marrow. Another signal from lining cells, capillaries, and platelets do the opposite and stimulate travel. Many complex interactions occur using multiple cytokines (IL-23, IL-17) with other immune cells including macrophages in the tissues and traveling dendritic cells.
G-CSF triggers genetic networks in stem cells, and to help send them into the blood. The great majority of these leukocytes are stored in the bone marrow. It inhibits receptors that hold them in the marrow. Another signal from lining cells, capillaries, and platelets do the opposite and stimulate travel. Many complex interactions occur using multiple cytokines (IL-23, IL-17) with other immune cells including macrophages in the tissues and traveling dendritic cells.
Some neutrophils are made outside of the bone marrow, such as the lungs via signaling.
Specific types of signals (eicosanoids and other cytokines) first call for neutrophils, monocytes, and macrophages to start inflammation after an attack or damage occurs. When many neutrophils die, signals tamp down the inflammation and alter the macrophages to do repair instead. Chronic inflammation occurs if the original assault continues (such as not killing the microbes or autoimmune attacks that continue) or by not being able to signal for the healing tamp down response.
Stress increases the production of neutrophils (granulopoiesis) and some of these processes occur in chronic situations. Special signals move the stem cells in the direction of the producing neutrophils. In states where cholesterol or blood sugars are very high for a long time (diabetes and blood vessel disease), these signals are also high. This adds other cytokines. Signals from the brain stress system (steroids and adrenalin from adrenal pituitary axis and sympathetic nervous system) also triggers more leukocytes. These increase damage of blood vessels with atherosclerosis plaques. Inflammation and leukocytes are both triggered together.
Aging Changes in Neutrophils
 One factor that alters neutrophils is aging and metabolism triggers changes in producing them and their functions. Defects occur in the signaling pathways inside of cells. The ability to travel is maintained but the response to signals for exact migration is altered. One pathway (PI3K) is altered, which alters migration. When this pathway was interfered with in research, the cells were able to travel accurately again.
One factor that alters neutrophils is aging and metabolism triggers changes in producing them and their functions. Defects occur in the signaling pathways inside of cells. The ability to travel is maintained but the response to signals for exact migration is altered. One pathway (PI3K) is altered, which alters migration. When this pathway was interfered with in research, the cells were able to travel accurately again.
They are also altered in their abilities to kill microbes with altered NETs and decreased ability to eat cells and debris. They also make more ROS and have less proteins enzymes released. There are also changes in energy.
A special process (neutrophil priming) increases normal neutrophils into fighting machines. This is triggered by many different signals. They make more ROS and change shape. Increased sugar in the blood causes more NETs to be released (from more ROS as well from the increased sugar). Multiple changes with high cholesterol and sugar alter their functions.
Conversations At Infection Site
 Neutrophils are very chatty at the inflammation site and in lymph tissues with platelets, lymphocytes, monocytes, and DCs.
Neutrophils are very chatty at the inflammation site and in lymph tissues with platelets, lymphocytes, monocytes, and DCs.
A previous post showed how platelet conversations with neutrophils are very important to start the inflammation. Platelets are also involved in chronic inflammation of arthritis, atherosclerosis, and bowel disease. Many different signals and receptors are exchanged. Platelets are vital to activate neutrophils with many signals. Some trigger NETs possibly causing more blood vessel plaque. Multiple back and forth signals are involved in providing enough neutrophils in the tissues for the job at hand.
In acute inflammation, there are mostly neutrophils but in chronic inflammation, monocytes and macrophages. Signals from neutrophils call monocytes. Some of the granule proteins are involved in calling for monocytes. Platelets help signal to neutrophils for this process.
There appear to be multiple sub types of these chronic cells but the research is just beginning. Some only live for a few hours thought to not have many variants in such a short time. There are those that support and inhibit inflammation. Some stop inflammation in tumors and help cancers. Some produce more ROS.
Netrophil Conversations with Macrophages About Dying
 Macrophages are the most prominent cell in chronic inflammation. They eat debris. Signals from neutrophils cause macrophages to be the type that makes inflammation. They respond to NETs also. NETs plus a lipid signal make macrophages and send many signals to cause inflammation. Some of these signals in turn help the neutrophils to live longer and stay at the site.
Macrophages are the most prominent cell in chronic inflammation. They eat debris. Signals from neutrophils cause macrophages to be the type that makes inflammation. They respond to NETs also. NETs plus a lipid signal make macrophages and send many signals to cause inflammation. Some of these signals in turn help the neutrophils to live longer and stay at the site.
By stopping the death of neutrophils, signals to tamp down and resolve inflammation are avoided. Dead neutrophils absorb many signals and they break down signals for inflammation. Macrophages eat up dead neutrophils when they send signals to find them and eat them. They then produce IL-10 to stop further. Not letting them die, avoids all of this activity.
Neutrophils that are dying, but not dead, increase inflammation. The entire elaborate process whereby macrophages find and eat dead neutrophils is undermined when they are only dying but still there. This increases and prolongs inflammation. Further, by interfering with this process, chronic inflammation becomes inadequate at clearing dead cells. ROS from neutrophils furthers this defective clearance. There are many aspects to the ways that macrophages normal clearance is interfered with.
As well, neutrophils travel in lymphatics and converse with lymphocytes and DCs. They go to the special zones of lymph tissue signaling to T cells.
Chronic Inflammation
 There has been much research on neutrophils in chronic inflammation helping cancer development, arthritis, and lung disease. New research shows involvement in metabolic disease such as obesity and diabetes.
There has been much research on neutrophils in chronic inflammation helping cancer development, arthritis, and lung disease. New research shows involvement in metabolic disease such as obesity and diabetes.
In obesity, macrophages promote inflammation in fat tissues. But, neutrophils are also called there with high fat diet. These appear to help the macrophages. They appear in liver and decrease insulin receptors.
In brain degeneration, amyloid plaque increases monocytes and microglia with neuro inflammation. Neutrophils also are in plaques and in the blood vessels nearby attached to the lining cells. It is possible that without neutrophils, cognitive impairment is less. With less, there were less microglia as well. NETs and IL-17 can directly hurt neurons.
Neutrophils are involved in starting blood vessel plaque disease. Close to plaques, they sit in between capillaries and muscle cells. In plaques, they are near macrophages and micro vessels of large advanced lesions. With more, there is more heart disease later.
Wound Healing
 Inflammation is involved in successful healing and limb regeneration but also scarring and production of cancer (see post). All of these are part of processes from the phase of inflammation related to tamping down of efforts. Recent research shows neutrophils helping after spinal cord injury and in the lungs. Specific programs appear to be involved such as unique genetic networks for specific microbes. They help stimulate new blood vessels and oxygen supply. They alter macrophage activity changing them to resolution of inflammation types. They release several signals to make new vessels. Cancer cells hijack these signals to make vessels for themselves.
Inflammation is involved in successful healing and limb regeneration but also scarring and production of cancer (see post). All of these are part of processes from the phase of inflammation related to tamping down of efforts. Recent research shows neutrophils helping after spinal cord injury and in the lungs. Specific programs appear to be involved such as unique genetic networks for specific microbes. They help stimulate new blood vessels and oxygen supply. They alter macrophage activity changing them to resolution of inflammation types. They release several signals to make new vessels. Cancer cells hijack these signals to make vessels for themselves.
In addition to increasing plaque, neutrophils help after heart attack and surgery for heart blood vessels. They come to the artery and stop an accumulation of a large amount. They both promote the inflammation and stop it. Like many aspects of conversations among cells they can go both ways. Too many cells cause problems after heart attack.
Neutrophils also go both ways in the intestine inflammations of colitis, Crohn’s and Inflammatory Bowel Disease (IBD). Different models provide different results. They remove debris and bacteria with molecular toxins. Polypeptide products help repair by calling for more lining epithelial cells and more mucous. Special molecules from neutrophils call for more lining cells. They also call for mesenchymal cells that have been described in other posts as more dynamic connective structural cells to help repair of tissue. They also signal to macrophages to tamp down their damaging behavior. They educate the macrophages as they eat up dead neutrophils. In inflammatory bowel disease there are less of these lipid signals and these have been used in treatments.
Treatment of Chronic Inflammation
I n fact, many of these signaling pathways are being evaluated for intervention with treatments. Finding ways to tamp down chronic inflammation with neutrophils can provide important new treatments. The key is to find signals that only affect a particular organ, so as to not stimulate more infections elsewhere with less neutrophils.
n fact, many of these signaling pathways are being evaluated for intervention with treatments. Finding ways to tamp down chronic inflammation with neutrophils can provide important new treatments. The key is to find signals that only affect a particular organ, so as to not stimulate more infections elsewhere with less neutrophils.
One tactic is to stop NETs that keep inflammation going in blood vessel disease and lung disease. Stopping platelet signals to these NETs appears promising. To make NETs, chromatin has to be altered and condensed. Altering charge on some of the proteins involved are another way to stop the process.
Another approach is to stop the signals that call for neutrophils. This approach is hampered by the complexity of the signals involved where those calling for more cells are similar to other molecules in completely different processes. Research is attempting to find very specific molecules that will only affect one organ.
Therapies are also aimed at those signals that tamp down inflammation behavior directly, which seem to affect all aspects of the processes of calling for these cells and maintaining them and then getting rid of them. One chemical is taken from Chinese herb (tanshinone IIA) that tells neutrophils to leave the inflamed area. Others increase programmed suicide in several different ways. Statins seem to increase macrophage clearance of dead neutrophils in artery plaque.
Neutrophils produce very small sacs (microvesicles) with proteins and nucleic acids. They tend to stop inflammation with cytokines and triggering cells to tamp it down. Treatments now are attempting to use them as well.
White Blood Cell Conversations In Chronic Inflammation
 As cellular conversations are discovered, cellular behavior appears more complex. Now, white blood cells that are called to acute infections appear to have multiple different phases of activity with support from many other cells via signals for each phase. There is the call to travel to an acute infection, and even production of more cells in bone marrow. There is crossing from the blood into tissue and the many different behaviors to produce inflammation types that rid the body of various microbes and repair damage in many different ways.
As cellular conversations are discovered, cellular behavior appears more complex. Now, white blood cells that are called to acute infections appear to have multiple different phases of activity with support from many other cells via signals for each phase. There is the call to travel to an acute infection, and even production of more cells in bone marrow. There is crossing from the blood into tissue and the many different behaviors to produce inflammation types that rid the body of various microbes and repair damage in many different ways.
It was recently found that some of these leukocytes then tamp down the inflammation and some are instructed to travel elsewhere to continue work. Now, the conversations of abnormal cells producing chronic inflammation diseases have been found. One is related to the programmed death of cells that have completed their work. If they die, then dead cells send signals to tamp down inflammation. If they are dying but not dead, signals are for continued and abnormal chronic inflammation. Understanding these conversations will allow future treatments.