 The original definition of inflammation included four symptoms—fever, redness, heat, and pain—and only pain was associated with neuronal signals. Recently, it was shown that, in fact, neuronal activity is involved in mediating all four inflammation symptoms (See post). But, in fact, inflammation is far more complex than previously known, with a very large number of different immune cells and brain cells signaling back and forth with cytokines and neurotransmitters causing many different types of activity. In fact, neurons can by themselves produce all the actions of inflammation and use inflammation pathways in neuroplasticity.
The original definition of inflammation included four symptoms—fever, redness, heat, and pain—and only pain was associated with neuronal signals. Recently, it was shown that, in fact, neuronal activity is involved in mediating all four inflammation symptoms (See post). But, in fact, inflammation is far more complex than previously known, with a very large number of different immune cells and brain cells signaling back and forth with cytokines and neurotransmitters causing many different types of activity. In fact, neurons can by themselves produce all the actions of inflammation and use inflammation pathways in neuroplasticity.
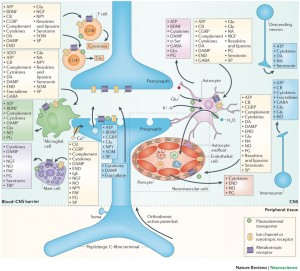
Neuronal inflammation refers to inflammation that occurs solely because of neuron activity, not in response to microbes and damage. The stimulation of inflammation pathways by neurons is now, also, known to be critical for  neuroplasticity—yet another mechanism. What is even more remarkable is that neuronal synapses in inflammation pathways are not typical connections between pre synaptic and post synaptic neurons (surrounded by a supporting astrocyte), but are, actually, at least a six part synapse involving a pre synaptic neuron signaling to other neurons, astrocytes, microglia, macrophages, vascular lining cells and T cells. The extraordinary complexity of the neuron inflammation pathways is just beginning to be understood. The inflammation pathways in neuroplasticity are involved in cognition, learning, pain, and other brain activity.
neuroplasticity—yet another mechanism. What is even more remarkable is that neuronal synapses in inflammation pathways are not typical connections between pre synaptic and post synaptic neurons (surrounded by a supporting astrocyte), but are, actually, at least a six part synapse involving a pre synaptic neuron signaling to other neurons, astrocytes, microglia, macrophages, vascular lining cells and T cells. The extraordinary complexity of the neuron inflammation pathways is just beginning to be understood. The inflammation pathways in neuroplasticity are involved in cognition, learning, pain, and other brain activity.
What is Inflammation?
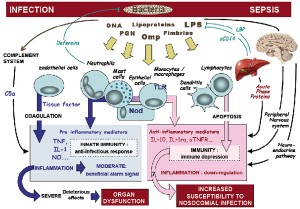 In addition to the four original symptoms, inflammation now also includes complex interactions between immune and brain cells, antigens and the complement system, and T cell reactions, which can lead to tissue death and scarring. Inflammation can protect and harm at the same time. While essential for protection against dangerous microbes it contributes to many chronic diseases.
In addition to the four original symptoms, inflammation now also includes complex interactions between immune and brain cells, antigens and the complement system, and T cell reactions, which can lead to tissue death and scarring. Inflammation can protect and harm at the same time. While essential for protection against dangerous microbes it contributes to many chronic diseases.
 Making inflammation even more complex, brain signals stimulate fever, sleep and multiple types of pain. The fever helps kill microbes; recent research shows that taking anti fever medications might prolong the effect of cold viruses. Pain and sleep help rest to allow healing. A previous post showed that T cells send signals to help cognition in normal conditions and then send signals to decrease cognition when fighting a microbe.
Making inflammation even more complex, brain signals stimulate fever, sleep and multiple types of pain. The fever helps kill microbes; recent research shows that taking anti fever medications might prolong the effect of cold viruses. Pain and sleep help rest to allow healing. A previous post showed that T cells send signals to help cognition in normal conditions and then send signals to decrease cognition when fighting a microbe.
Neurons Cause Brain Inflammation
Brain inflammation can be a reaction to microbes, toxins, trauma and neural degeneration. Neuronal activity, alone, can cause inflammation in the brain, and use it for many different purposes. A complex network of immune cells and cells that line blood vessels produce factors that increase inflammation to fight disease, but, also, are also activated by neurons for neuroplasticity and increased metabolism.
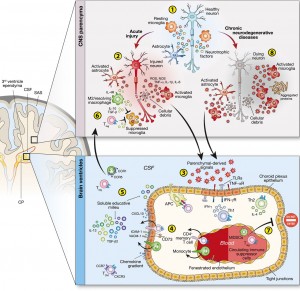 As with innate and adaptive immune responses, neurons can trigger mild tissue changes called “para-inflammation,” which are mainly adjustments, rather than full-blown inflammation with radical changes in the tissue. In the full inflammation response triggered by neurons, blood vessels open wide and pores allow large molecules, fluid and immune cells to leave the blood and enter the brain.
As with innate and adaptive immune responses, neurons can trigger mild tissue changes called “para-inflammation,” which are mainly adjustments, rather than full-blown inflammation with radical changes in the tissue. In the full inflammation response triggered by neurons, blood vessels open wide and pores allow large molecules, fluid and immune cells to leave the blood and enter the brain.
In the smaller “para” reactions, cellular activity can be used for these other neuroplasticity purposes. These smaller reactions may only be signaling to distant cells, sometimes triggering astrocytes, microglia, T cells and vascular cells. It can trigger coordinated actions of immune cells, vessel cells and neurons together. This activity can increase metabolism when needed, increasing the power of brain circuits and increasing neuroplasticity.
Versions of this partial  inflammatory pathway stimulated by neurons influence neuroplasticity, metabolism, axon potential, glutamate toxicity, degeneration and regeneration. Whether the effects are positive or negative depend upon the degree, the location and the specific signals.
inflammatory pathway stimulated by neurons influence neuroplasticity, metabolism, axon potential, glutamate toxicity, degeneration and regeneration. Whether the effects are positive or negative depend upon the degree, the location and the specific signals.
As a reaction to microbes or injury in the periphery, inflammation dilates blood vessels, allows fluid to leak, attracts immune cells, and releases a large number many substances, such as cytokines, prostaglandins, serotonin, histamine, inflammatory factors. All of this leads to sensitized pain fibers and pain.
The same process occurs in the brain with neuron stimulation causing activation of immune cells, vascular cells, and other neurons causing pain in the spinal cord. The factors involved are glutamate, substance P, calcitonin gene related peptide (CGRP) and brain derived neurotrophic factor (BDNF), fractaline and ATP.
Blood Vessels Play Important Role
 When neuronal activity increases in a stressed situation, there is a need for more metabolic activity. The inflammatory response from the neuron will increase blood flow. Blood vessels respond to inflammatory stimuli and toxins by opening and sending more blood. A signaling network of neurons, astrocytes and endothelial cells that line the vessels regulate the response of the vessels.
When neuronal activity increases in a stressed situation, there is a need for more metabolic activity. The inflammatory response from the neuron will increase blood flow. Blood vessels respond to inflammatory stimuli and toxins by opening and sending more blood. A signaling network of neurons, astrocytes and endothelial cells that line the vessels regulate the response of the vessels.
 Endothelial cells, like neurons, T cells, astrocytes, and microglia are very active, intelligent cells that send and receive many different signals with immune cells and brain cells. One type of endothelial cell lines blood vessels and another type lines the choroid plexus and secretes cerebrospinal fluid.
Endothelial cells, like neurons, T cells, astrocytes, and microglia are very active, intelligent cells that send and receive many different signals with immune cells and brain cells. One type of endothelial cell lines blood vessels and another type lines the choroid plexus and secretes cerebrospinal fluid.
Neuronal stimulation that is moderate can open blood vessels; stimulation that is very strong can open the blood brain barrier. All of these different cells—neurons, microglia, astrocytes, immune cells and the blood vessel lining cells—can signal to open the vessels and send more blood.
A Very Complex Synapse
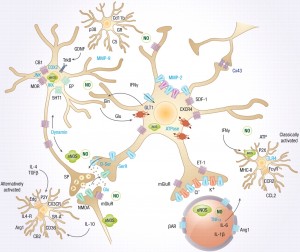
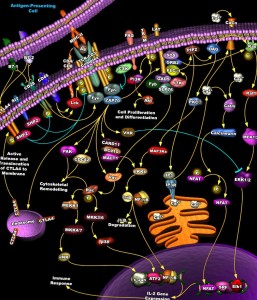 Neurons have a back and forth dialogue with other neurons. But, they also have chatter with microglia, astrocytes, T cells, endothelial cells and even the extracellular matrix (see post on neuroplasticity of ECM).
Neurons have a back and forth dialogue with other neurons. But, they also have chatter with microglia, astrocytes, T cells, endothelial cells and even the extracellular matrix (see post on neuroplasticity of ECM).
Instead of the well-known synapse between two neurons this virtual synapse involves multiple neurons, microglia, astrocytes, T cells, macrophages, and endothelial cells in blood vessels and the choroid plexus, at least.
One example is when NK1 receptors in the spinal cord neuron cause neuroplasticity through long term potentiation, LTP. At the same time NK1 has similar effects on astrocytes, microglia, vascular lining cells and T cells. All of this contributes to inflammatory activity of various types that, also, contributes to neuroplasticity in the neuronal circuit.
Cytokines can, also, have multiple effects, which are synergistic. IL-1 receptors on dorsal horn neurons, glial cells, and endothelial cells stimulate prostaglandin release through COX1 and COX2. All of these can stimulate glutamate, substance P and CGRP.
Both Inflammation and Anti Inflammation At the Same Time
 There are examples where the multiple effects oppose one another, causing inflammation and blocking inflammation. Neurons have different effects at low, medium and high activity. Moderate activity of neurons cause anti-inflammatory reactions.
There are examples where the multiple effects oppose one another, causing inflammation and blocking inflammation. Neurons have different effects at low, medium and high activity. Moderate activity of neurons cause anti-inflammatory reactions.
An example is glutamate mGluR5 receptors on neurons, astrocytes, microglia, T cells and endothelial cells. These stimulate LTP at a synapse with increased calcium in neurons, microglia and astrocytes. But, the same stimulation in spinal microglia inhibits the inflammatory signals. In one region it causes long-term depression, LTD, instead of LTD.
When immune T cells trigger inflammation, they, also, trigger anti-inflammatory factors, so the inflammation can be controlled without too much damage. Neurons, also, stimulate both inflammation and anti-inflammation behavior in T cells and microglia.
 Some cytokines have both pro inflammatory and anti-inflammatory actions in different circumstances and regions. As an example fractalkine is pro inflammatory in the spinal dorsal horn causing pain; it is anti inflammatory in the cortex in the context of Parkinson’s.
Some cytokines have both pro inflammatory and anti-inflammatory actions in different circumstances and regions. As an example fractalkine is pro inflammatory in the spinal dorsal horn causing pain; it is anti inflammatory in the cortex in the context of Parkinson’s.
Because of these complex effects, it is possible that many drugs currently used operate in these inflammatory pathways, as opposed to currently accepted mechanisms. For example, COX inhibitors, antipsychotics, epilepsy drugs and cannabinoids all reduce neuroinflammation.
Neuroplasticity and Inflammation
 The many mechanisms of neuroplasticity allow adaptation to changing circumstances, such as altered communication between neurons with long-term potentiation (LTP). (See post for many other neuroplasticity mechanisms). Inflammatory pathways triggered by neurons alone affect neuroplasticity in pain, psychological stress, migraine, sleep, learning, mood, autism and epilepsy. Inflammation pathways from neuronal stimulation include many signals, such as BDNF, ATF, TNF and IL, which are essential for neuroplasticity.
The many mechanisms of neuroplasticity allow adaptation to changing circumstances, such as altered communication between neurons with long-term potentiation (LTP). (See post for many other neuroplasticity mechanisms). Inflammatory pathways triggered by neurons alone affect neuroplasticity in pain, psychological stress, migraine, sleep, learning, mood, autism and epilepsy. Inflammation pathways from neuronal stimulation include many signals, such as BDNF, ATF, TNF and IL, which are essential for neuroplasticity.
Ordinary inflammation and neuron stimulated inflammation both constantly interact. Inflammation from neurons occurs in specific localized regions and in graded intensity, with different effects in different locations and at different levels. If neuron signals are very strong, it stimulates classical inflammation with fluid and immune cells entering the brain tissue. There is really no line between classical and neuron stimulated inflammation, but degrees.
Inflammatory stimulation by neurons can be too strong. Even small injuries can lead to abnormal neuronal activity and lead to chronic inflammation and pain.
 Neuroplasticity in Pain: Neuroplastic long-term potentiation changes occur in the pain fibers causing increased pain sensitivity. If the cause of the pain continues, such as a damaged nerve (neuropathy) there are alterations that become abnormal—changes in the blood brain barrier leads to new immune factors that are unique and increase inflammation or fight against inflammation. These factors can spread to a larger region. When things get worse there is flow of fluid, large molecules and other immune cells into the nervous system tissue which can further damage neurons. In this case, neurons from the higher brain will stimulate even more inflammation through immune cells. Glial cells are not able to keep up buffering the increased glutamate and this leads to more toxicity and increased pain in the adjacent areas.
Neuroplasticity in Pain: Neuroplastic long-term potentiation changes occur in the pain fibers causing increased pain sensitivity. If the cause of the pain continues, such as a damaged nerve (neuropathy) there are alterations that become abnormal—changes in the blood brain barrier leads to new immune factors that are unique and increase inflammation or fight against inflammation. These factors can spread to a larger region. When things get worse there is flow of fluid, large molecules and other immune cells into the nervous system tissue which can further damage neurons. In this case, neurons from the higher brain will stimulate even more inflammation through immune cells. Glial cells are not able to keep up buffering the increased glutamate and this leads to more toxicity and increased pain in the adjacent areas.
Neuronal inflammation can cause many types of pain. This inflammation can spread to other regions through astrocyte networks, which causes pain in adjacent or even distant areas, even in the opposite hemisphere. It can cause generalized pain, spontaneous pain, radiating pain and chronic pain.
 Increased pain can be useful at first to cause better protection of the injury. But, the same pain becomes a problem if it goes on for too long or if the pain spreads to inappropriate regions. The regulation of neuronal stimulation of too little or too much of the inflammatory pathways is very delicate.
Increased pain can be useful at first to cause better protection of the injury. But, the same pain becomes a problem if it goes on for too long or if the pain spreads to inappropriate regions. The regulation of neuronal stimulation of too little or too much of the inflammatory pathways is very delicate.
Neuroplasticity in Stress: Mental stress includes not just inflammation caused by neuronal activation, but increased hormones that affect in all regions of the body. Psychological stress can lead to increased immune cells and fluid entering the brain tissue. If this stress continues it leads to more inflammatory factors, increased activity of astrocytes and microglia. Genes can produce more nitric oxide and more COX2. Neuroplastic changes lower the pain thresholds and disrupt the blood brain barrier.
Neuroplasticity in Epilepsy: Seizures lead to many cytokine signals but not typical manifestations of inflammation. However, during epilepsy immune cells, vascular cells and neurons all take part in inflammatory actions. Seizures cause increased immune signaling, especially TNFa, IL-1b and IL-6 and MHC in the brain. Even one seizure can cause alterations in the blood brain barrier with immune cells and factors released into the brain.
Inflammation Doesn’t Just Recede – It is Actively Terminated
 Inflammation can become progressive in neurodegenerative disorders. Resolving inflammation is a multi step process that utilizes many different immune cells.
Inflammation can become progressive in neurodegenerative disorders. Resolving inflammation is a multi step process that utilizes many different immune cells.
Recent research shows that the cells in the choroid plexus play a crucial role in regulating the termination of inflammation in the brain, as well as the entire work of immune cells in the CSF. The choroid plexus, CP, has been long known to be a region of lining cells in the brain’s ventricles that secretes the fluid for the cerebral spinal fluid, CSF, which bathes the brain. CSF is the crucial region where T cells patrol and influence cognition with signaling.
Only recently, it has become clear that the CP is able to regulate the signals from the brain and the immune cells forming a gate for specific immune cells to enter the brain. In pathology this gate may become part of neurodegenerative disease with each disease having different types of inflammation.
 Through signaling, the CP recruits specific T cells to orchestrate the decrease of inflammation. What is remarkable about this “protective immunity” is that the CP can do this for any region of the brain even those quite far away.
Through signaling, the CP recruits specific T cells to orchestrate the decrease of inflammation. What is remarkable about this “protective immunity” is that the CP can do this for any region of the brain even those quite far away.
Each brain disease has different pathology and the CP is able to vary the response to these different forms of inflammation in different locations. In one situation microglia are activated to remove mis folded proteins and toxins (previous post). T cells patrol the CNS (see previous post) helping with cognition and neuroplasticity and the CP triggers specific T cells for anti-inflammatory activity.
These processes can help, but can, also, make matters worse in a vicious cycle.
In Alzheimer’s disease, amyloid beta is local and activates resting local microglia. They can stop formation of AB and clear it. However, the longer the microglia are active the more inflammation is increased. Also, macrophages called by the CP from outside of the brain attempt to decrease amyloid inflammation and neuron stimulated inflammation, with a decrease in cytokines, and more neutrophic factors.
In ALS the macrophages coming to clean up the inflammation, in fact, increase microglia activity and toxic inflammation particles in a vicious cycle of microglia making matters much worse.
Choroid Plexus Signaling Brings In Cells
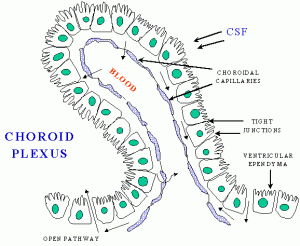 The macrophages coming to help have great difficulty getting into brain tissue because of the blood brain barrier. The CP is one layer of special, very intelligent, cells that have very unique qualities including immune surveillance of the CSF and mediating attraction of leukocytes to the CNS following damage. The CP is the gate for T cells and other immune cells to enter into the CSF and brain.
The macrophages coming to help have great difficulty getting into brain tissue because of the blood brain barrier. The CP is one layer of special, very intelligent, cells that have very unique qualities including immune surveillance of the CSF and mediating attraction of leukocytes to the CNS following damage. The CP is the gate for T cells and other immune cells to enter into the CSF and brain.
For the CP to recruit appropriate cells it uses elaborate signaling with many different cytokines. Different types of T cells interact with the CP and cause recruitment of appropriate different immune cells—some causing inflammation and some stopping it. The CP is the central place that allows anti-inflammatory cells into the brain and determines which cells would help specific different types of inflammation in degenerative disease.
Inflammation Pathways in Neuroplasticity
 The intelligence of each of the cells involved in this new understanding of inflammation is extraordinary. Neurons communicate not only with large circuits of neurons, but to synapses involving many other intelligent cells all sending wireless signals back and forth. The neuron, also, can grade its signals to the other neurons, astrocytes, microglia, T cells and cells lining the blood vessels and choroid plexus. The different levels of stimulation at specific locations cause very different types of inflammation activity. Some moderate types of stimulation are used for neuroplasticity involving learning, pain, cognition, sleep, epilepsy and more. These signals can vary according to the circumstances.
The intelligence of each of the cells involved in this new understanding of inflammation is extraordinary. Neurons communicate not only with large circuits of neurons, but to synapses involving many other intelligent cells all sending wireless signals back and forth. The neuron, also, can grade its signals to the other neurons, astrocytes, microglia, T cells and cells lining the blood vessels and choroid plexus. The different levels of stimulation at specific locations cause very different types of inflammation activity. Some moderate types of stimulation are used for neuroplasticity involving learning, pain, cognition, sleep, epilepsy and more. These signals can vary according to the circumstances.
Previous posts have described the unusual intelligence of T cells, microglia and astrocytes. But, the lining cells of the choroid plexus are just as unusual. These cells are the gateways of signaling and the immune cells’ entrance into the brain. CP cells can determine where and what type of inflammation is occurring and modify the response to that specific region at a distance and to the specific disease process.
The communication involved in these processes, whereby inflammation pathways are utilized for metabolism and neuroplasticity, is so complex that it is hard to not consider these cells as intelligent. Is it possible that the mind finds ways to use any mechanism it can for neuroplasticity?