Recently, experiments were able to erase drug-associated memories in mice without affecting other memories. This may one day help humans with unwanted memories in posttraumatic stress. What is remarkable is that this was accomplished using a new form of neuroplasticity. Inhibition of the specific myosin motor II during the memory altered the actin structures in the neurons involved in memory. This post is about yet another new type of neuroplasticity with myosin motors.
Recently, experiments were able to erase drug-associated memories in mice without affecting other memories. This may one day help humans with unwanted memories in posttraumatic stress. What is remarkable is that this was accomplished using a new form of neuroplasticity. Inhibition of the specific myosin motor II during the memory altered the actin structures in the neurons involved in memory. This post is about yet another new type of neuroplasticity with myosin motors.
Thought, somehow, instantly creates complex molecular changes throughout widely distributed brain circuits. How thought is connected to these forms of neuroplasticity is not known. But, remarkably, there are an increasing number of ways that this occurs. It appears that thought can somehow change  structures through stimulation of new proteins with new shapes, by triggering DNA, then new editing of the messenger RNA transcripts. Somehow, the cell knows what these new shapes will do when interlocking with all the other existing molecules. Through this process very complex motors are built, like the dynein, kinesin and myosin motors. The language of shapes appears to be the way neurons know what to do when mental events occur. The constantly changing structures appear to be the language of the cell. Previous posts have shown many different mechanisms by switching receptor subunits, switching neurotransmitters and entire receptors and many others. Here we describe yet another new type of neuroplasticity with myosin motors.
structures through stimulation of new proteins with new shapes, by triggering DNA, then new editing of the messenger RNA transcripts. Somehow, the cell knows what these new shapes will do when interlocking with all the other existing molecules. Through this process very complex motors are built, like the dynein, kinesin and myosin motors. The language of shapes appears to be the way neurons know what to do when mental events occur. The constantly changing structures appear to be the language of the cell. Previous posts have shown many different mechanisms by switching receptor subunits, switching neurotransmitters and entire receptors and many others. Here we describe yet another new type of neuroplasticity with myosin motors.
Myosins are complex protein motors that provide many functions for neuroplastic structural changes by working with the scaffolding molecules actin. These motors use cellular energy to build, break down and rebuild structures. A previous post, the Remarkable Microtubule, described the LEGO language of scaffolding molecules in the neurons. In fact, it is now known that there are a wide range of different motors for different purposes.
Neuroplasticity with Many Mechanisms
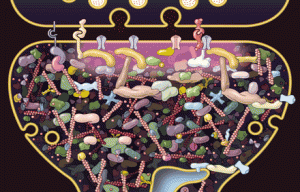 Synaptic neuroplasticity is the basis of all cognitive processes. The process involves building, breaking down and rebuilding synapses, both intra and external cellular structures. Strengthening and weakening of the synapses are critically related to pre synaptic active zones where vesicles with neurotransmitters are launched, and dendrite spines that receive the input. Both grow and shrink based upon the representation of thought. Before a totally new type of mechanism is described the following is a list of previously described different mechanisms (See previous posts for more details on each):
Synaptic neuroplasticity is the basis of all cognitive processes. The process involves building, breaking down and rebuilding synapses, both intra and external cellular structures. Strengthening and weakening of the synapses are critically related to pre synaptic active zones where vesicles with neurotransmitters are launched, and dendrite spines that receive the input. Both grow and shrink based upon the representation of thought. Before a totally new type of mechanism is described the following is a list of previously described different mechanisms (See previous posts for more details on each):
- Manufacture and placement of more AMPA glutamate receptors
 Postsynaptic density alters structure (over 1000 interlocking large complex proteins)
Postsynaptic density alters structure (over 1000 interlocking large complex proteins)- Alterations of molecules that stick out and hold together neurons at the synapse (neuroligins and neurexins)
- Calcium surge triggers new types of “memory proteins”
- Substitution of new matching neurotransmitters and receptors
- Alteration of signaling cascades from the membrane to the nucleus
- Changes in axon ion channels altering electrical signal
- Change in balance of inhibition and stimulation
 New micro RNA’s alter process
New micro RNA’s alter process- Interneurons altered
- Mitochondria change strength of the signal.
- Focused attention changes synapse structure
- Climbing fibers in cerebellum with multiple alterations
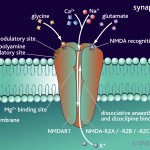
- NMDA glutamate receptors substitute their subunits
- Transport motors are substituted
- Actin and microtubules direct scaffolding structural changes
- Exosomes send information from astrocytes to neurons including pieces of DNA and proteins
Scaffolding Tubules
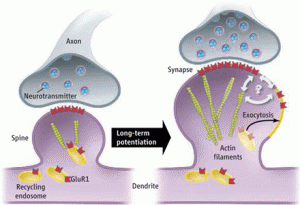 There are thousands of different types of scaffolding molecules in human cells. With neuroplasticity these enlarging and condensing molecules are constantly and instantly building structures for the budding dendrites, the progressing axons and their boutons which attach to the dendrites, and the massive transit systems throughout the neuron.
There are thousands of different types of scaffolding molecules in human cells. With neuroplasticity these enlarging and condensing molecules are constantly and instantly building structures for the budding dendrites, the progressing axons and their boutons which attach to the dendrites, and the massive transit systems throughout the neuron.
In neurons there are three basic important types of building blocks, each with different capacities that work together for the many rapid difficult scaffolding jobs. With these three molecules thousands of different complex lattice structures can be built. The 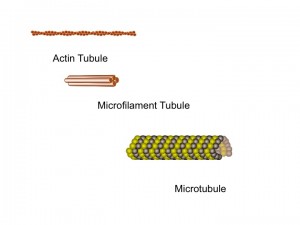 first molecule, actin, which is also used in muscle, can build a membrane’s moving edge. Actin comes into play when a neuron grows a dendrite or axon.
first molecule, actin, which is also used in muscle, can build a membrane’s moving edge. Actin comes into play when a neuron grows a dendrite or axon.
The simplest, most flexible scaffolding molecules are called microfilaments or intermediate filaments, which have branching capabilities that maintain their molecules’ flexibility and strength.
A previous post (The Remarkable Scaffolding Microtubule) described the largest and strongest structural scaffolding element, the microtubule. The microtubule has a 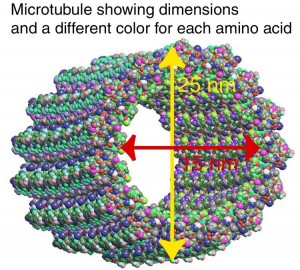 unique helical shape and is critically important in the neuron. Inside this microtubule helix, with a diameter of 25 nanometers, the local environment is determined by the specific amino acids in the proteins. Because of the unique physical structure inside these microtubules, it has been proposed that a form of biological computer could be directing neuroplasticity from this space. It is certainly remarkable how tubules know how to rapidly create and destroy structures as neuroplasticity occurs.
unique helical shape and is critically important in the neuron. Inside this microtubule helix, with a diameter of 25 nanometers, the local environment is determined by the specific amino acids in the proteins. Because of the unique physical structure inside these microtubules, it has been proposed that a form of biological computer could be directing neuroplasticity from this space. It is certainly remarkable how tubules know how to rapidly create and destroy structures as neuroplasticity occurs.
Motors in Neurons
Kinesins are a group of at least fourteen different proteins that “walk” along  microtubules. They transport mitochondria, Golgi bodies, and vesicles carrying cargo. They can walk from the plus or minus end of the microtubule. They have two chains with motor heads that walk in an asymmetric.
microtubules. They transport mitochondria, Golgi bodies, and vesicles carrying cargo. They can walk from the plus or minus end of the microtubule. They have two chains with motor heads that walk in an asymmetric.
Dynein are a group of 17 different very large complex motors, larger than Kinesin and the myosin motors. They move along microtubules with a sliding motion. They are critical in cilia and flagella.
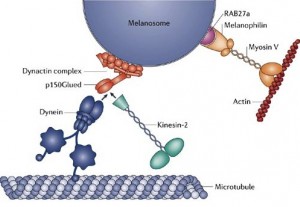 Myosins motors move on actin complexes (with a simpler structure than microtubules), and are most well known to move muscle. But, they have many other purposes including cellular division, streaming of organelles in the cytoplasm and neuroplasticity in the brain. In some unusual circumstances myosin motors on actin tracks work together cooperatively at the same time with dynein and kinesin motors to carry a large complex load (see picture above).
Myosins motors move on actin complexes (with a simpler structure than microtubules), and are most well known to move muscle. But, they have many other purposes including cellular division, streaming of organelles in the cytoplasm and neuroplasticity in the brain. In some unusual circumstances myosin motors on actin tracks work together cooperatively at the same time with dynein and kinesin motors to carry a large complex load (see picture above).
Myosin Motors are Critical for Neuroplasticity
The small tracks created by actin are critical in neuroplasticity with rapid scaffolding changes in growing axons, dendrites budding and the very subtle changes that occur with remodeling of synapses.
Most people think of myosin as the motor of our muscles. But, in fact, there are 35 different classes of myosin and several important ones are critical in neuroplasticity.
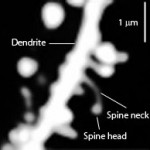
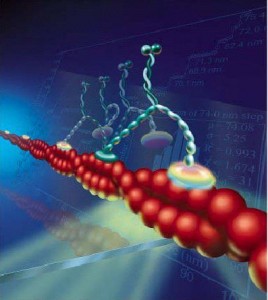 Myosin depends upon scaffolding structures based upon actin filaments. Actin has a barbed plus end and a pointed minus end. Dendrite spine heads and necks have linear and branched actin filaments, which are highly changeable. They are constantly growing into a polymer with many attached actin molecules and then shrinking by shedding some of them. These changes occur in seconds. The actin determines how fast the neurotransmitter is diffused.
Myosin depends upon scaffolding structures based upon actin filaments. Actin has a barbed plus end and a pointed minus end. Dendrite spine heads and necks have linear and branched actin filaments, which are highly changeable. They are constantly growing into a polymer with many attached actin molecules and then shrinking by shedding some of them. These changes occur in seconds. The actin determines how fast the neurotransmitter is diffused.
In the presynaptic terminal actin can facilitate the release of vesicles by attaching them to the membrane or block release. The actin knows to build a blockage when the message isn’t being sent.
A Lot of Different Myosins
Myosins are large protein molecules that attach to actin structures. These act as enzymes and provide movement and force by using ATP made from mitochondria. Myosins propel actin filament movement but also walk along the actin structures. Myosins determine cellular transport of materials.
There are 35 classes of myosin motors, made by at least 40 different genes. Of these, the most is known for three classes, II, V and VI.
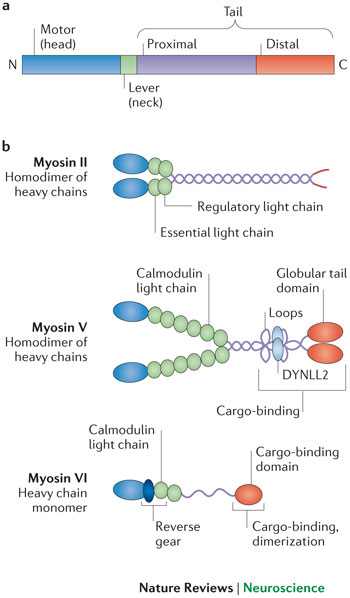 Class II myosins have six parts, two heavy chains, and four light chains (two essential and two regulatory). These can be in skeletal and cardiac muscle causing muscular force. There are also non-muscle myosins in this class that help cell migration and protrusion and adhesion with integrin and cadherin. It is also critical in the actomyosin ring during cell division.
Class II myosins have six parts, two heavy chains, and four light chains (two essential and two regulatory). These can be in skeletal and cardiac muscle causing muscular force. There are also non-muscle myosins in this class that help cell migration and protrusion and adhesion with integrin and cadherin. It is also critical in the actomyosin ring during cell division.
Class V myosin is a motor that transports cargoes in the cell. It is made of chains of individual molecules binding six calmodulin light chains. It has a large step size, which means it can walk along one side of the helical actin track, not needing to spiral around the track. Va and Vb motors can move for a long distance on an actin track.
Class VI myosins walk in a different direction, that is, toward the actin’s pointed end. It has a reverse gear that is different from other motors. It has different linker proteins called cargo adaptors. It can sense the type of load and behaves differently with different types of loads. It can walk for a long time or anchor a heavy load to the actin.
Class VI is critical in launching vesicles with clathrin endocytosis. It is critical for movement of vesicles especially from the Golgi organelle, for fusion with the membrane, and to get vesicles to phagosomes. It is also a major part of many different cells, such as inner ear hair cells where it anchors and generates tension with the membrane and the cytoskeleton. People without the gene for myosin VI have deafness and vestibular circling behavior.
Three Myosins in the Brain
The three types of myosin II are in the brain (IIa, IIb, IIc). Type IIc is in neurons and blood vessels. Alternative RNA splicing creates multiple versions of b and c which have different properties.
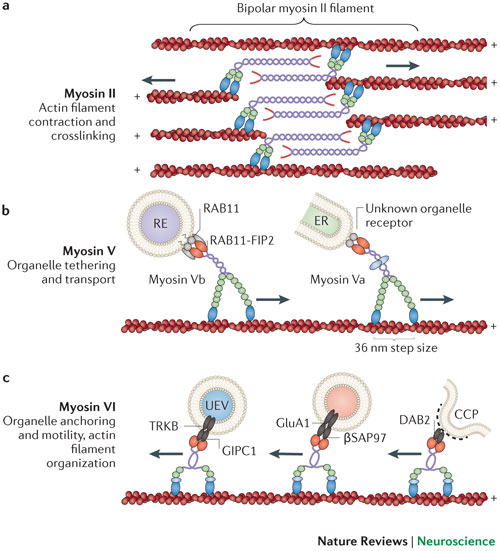 Synapses: Type b is in pre and post synaptic cells, especially in dendritic spine necks and heads and the post synaptic density.
Synapses: Type b is in pre and post synaptic cells, especially in dendritic spine necks and heads and the post synaptic density.
Dendrites: Myosin IIb regulates the shape and the building and rebuilding of dendritic spines in the hippocampus, the critical place for neuroplasticity of learning. It is IIb that allows the spine head of the NMDA receptor (see post for discussion of this critical neuron for all cognitive processes) to be enlarged into a “mushroom like dendritic spine head” with long term potentiation.
Myosin IIb has multiple different properties that can occur independently such as translocation and crosslinking. These processes are triggered by signals from phosphorylation. In fact, other functions occur by alternate splicing where these properties are disabled and the motors can serve other functions such as in cerebellar Purkinje cells crosslinking with actin creating tension. This somehow is related to functional dendritic spines.
Another distinct function occurs with a different alternate splicing of IIb in the hippocampus, somehow, creating a uniquely shaped dendritic spine head with different protrusions.
Post Synaptic Plasticity Maintained in Other Ways by Myosin
Myosin demonstrates many complex, surprisingly intelligent actions. Myosin stimulates actin to create longer additive molecules (polymerization) during LTP induction and myosin is then necessary to stabilize the new neuroplastic structures. On the parts of the synapse that protrude from the cell, such as the neuron’s growth cone, actin filaments grow on the leading end near the membrane, and disassemble those further away. Myosins exert force on actin structures to continue this flow of material from disassembly to the front leading edge. Myosin also cooperates with a number of other complex protein machines to build and rebuild these structures. Synaptic plasticity needs this coordinated actin/myosin machinery.
Neurotransmitter Release
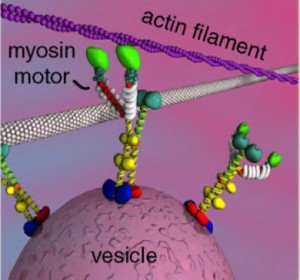 There are a wide range of critical myosin activities related to neurotransmitter release.
There are a wide range of critical myosin activities related to neurotransmitter release.
At the point of release of the neurotransmitters, myosins are required for the vesicles to release during electrical activity. It possibly does this by helping move the vesicles in the right direction to the membrane for release.
Two of three class V myosins are in the brain (Va is in many brain regions and Vb especially in the hippocampus). These myosin motors reside in dendritic spines and the post synaptic density. It appears that Vb must move along the actin filaments for LTP induction. To maintain neuroplasticity, myosin recycles the AMPA receptors that are manufactured in increased numbers. It also brings vesicles of material to the dendritic spines. When the recycled endosomes fuse with the membrane the AMPA receptor subunits are inserted into the membrane. As with other aspects of neuroplasticity this is determined by the amount of calcium.
Va is a transporter of organelles moving compartments from the ER along actin filaments to dendrite spines. This also is important in neuromuscular junctions. These motors carry secretory granules in endocine cells, and LDCV (large dense core vesicles) in the hippocampus.
VI is also in the brain and appears to mediate the effects of BDNF. It is also critical to transport of AMPA into the membrane.
Myosin Stepping
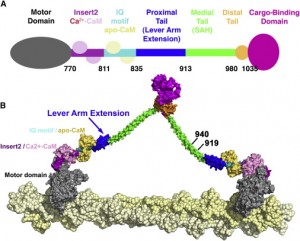 The size of the step for a myosin motor depends upon the lever that propels the motor forward. Myosin V lever is three times larger than II, V taking steps that are 36 nanometers long and II taking steps that are 7 nanometers long. To see an actual film of myosin walking click here.
The size of the step for a myosin motor depends upon the lever that propels the motor forward. Myosin V lever is three times larger than II, V taking steps that are 36 nanometers long and II taking steps that are 7 nanometers long. To see an actual film of myosin walking click here.
Myosin V moves in a hand over hand motion where the trailing foot leaves the actin and swings forward, just like humans walking. Myosin VI moves in the opposite direction also with 26 nm steps but also can take very small steps.
The motors are able to take many different steps because they are powered by ATP. When the ATP becomes ADP, it is released from the actin and is pushed forward by the lever motion. There is also an inherent strain on the molecules when both feet are attached that helps the motion forward. The timing of the release of release of ADP and binding of ATP are critical for the function of the stepping.
Yet Another New Type of Neuroplasticity with Myosin Motors
Myosin motors are very complex structures with at least seventeen families of different structures operating with the rapidly changing and very versatile actin strands that create the changing scaffolding structures to support neuroplasticity. These complex structures respond to subjective mental experience almost instantly, and are part of the language of scaffolding engineering and shape, that are the neuron’s response to mental events. Myosin motors demonstrate a very wide variety of seemingly intelligent actions, very surprising for a protein molecule.
Because they work with the actin molecules they are able to keep up with the constantly changing pre synaptic and post synaptic changes in the axon and the dendrite spines.
 There is no question that mental events stimulate these rapid millisecond changes in wide circuits, each brain region with different mechanisms. There are so many different mechanisms with so many different extremely complex proteins operating in the wide ranging neuroplastic circuits all at the same time, that it is difficult to imagine a central source other than the mind coordinating this. Somehow, the mind can act though a wide range of different molecular structures using the language of shape and function at both the molecular and circuit levels. It seems that wherever we look in the molecular structures there are new molecules responding to thought. Here we have yet another new type of neuroplasticity with myosin motors.
There is no question that mental events stimulate these rapid millisecond changes in wide circuits, each brain region with different mechanisms. There are so many different mechanisms with so many different extremely complex proteins operating in the wide ranging neuroplastic circuits all at the same time, that it is difficult to imagine a central source other than the mind coordinating this. Somehow, the mind can act though a wide range of different molecular structures using the language of shape and function at both the molecular and circuit levels. It seems that wherever we look in the molecular structures there are new molecules responding to thought. Here we have yet another new type of neuroplasticity with myosin motors.