 Brain and immunity cannot be separated. Almost every activity utilizes both. The previous post described dual functions of the most important molecules by the nervous and immune systems. Cytokines and neurotransmitters are signals for neurons, astrocytes and immune cells. Previous posts discussed the combined response to the internal threat of microbes. But, there is also an integrated combined response to human stress, social events in the external environment, and the critical survival responses to danger. Inflammation can be stimulated by microbe invasion, the stress of fight and flight or social loss. Positive mental and social events can trigger decrease in inflammation and increased attack on viruses. Thoughts not only trigger instantaneous molecular changes in widespread neuroplastic brain circuits, but, also, changes throughout the immune system. In fact, the brain and immunity fight internal and external foes together all of the time.
Brain and immunity cannot be separated. Almost every activity utilizes both. The previous post described dual functions of the most important molecules by the nervous and immune systems. Cytokines and neurotransmitters are signals for neurons, astrocytes and immune cells. Previous posts discussed the combined response to the internal threat of microbes. But, there is also an integrated combined response to human stress, social events in the external environment, and the critical survival responses to danger. Inflammation can be stimulated by microbe invasion, the stress of fight and flight or social loss. Positive mental and social events can trigger decrease in inflammation and increased attack on viruses. Thoughts not only trigger instantaneous molecular changes in widespread neuroplastic brain circuits, but, also, changes throughout the immune system. In fact, the brain and immunity fight internal and external foes together all of the time.
Both environmental experiences of danger and those of positive action have complex brain and immune responses. One recent study showed the complex  relationship of conscious human behavior and immunity. It showed a difference in gene expression in the immune system based on types of human activity and their enjoyment. Those who get pleasure from service to the community have a noted decrease in genes causing inflammation and increase of those fighting viruses. If the pleasure is from personal enjoyment, such as eating or hobbies, without community significance, there is no such benefit.
relationship of conscious human behavior and immunity. It showed a difference in gene expression in the immune system based on types of human activity and their enjoyment. Those who get pleasure from service to the community have a noted decrease in genes causing inflammation and increase of those fighting viruses. If the pleasure is from personal enjoyment, such as eating or hobbies, without community significance, there is no such benefit.
At the microscopic level special genes respond to many signals in the environment to create immune response including manufacture and secretion of antibodies cytokines, peptides to fight microbes, and molecules that can kill cells. These genes are activated by molecular patterns from microbes (called PAMPs – pathogen associated molecular patterns – see post ) and signals of danger from deteriorating cells, cancer cells, or cells that are stressed or are part of the pathways of cell death (apoptosis, autophagy).
At the level of the human being, hormones and neuronal signals also modulate immune activity and have major effects on immune cells. Feedback loops of immune signaling cytokines coordinate fight and flight responses throughout the body. These immune/nervous system responses not only fight microbes, but also are critical in repair after trauma, sleep loss, and also, reactions to violence, bullying, social isolation, and social or personal loss.
Triggering Immunity and Judging Most Important Reactions for Survival
 Immune responses are extremely complex because they are triggered both by the brain’s reaction to external and internal threats, social situations, as well as internal processes such as cellular damage, microbe invasion and cancer. The back and forth communication of brain and immunity make all of these responses much more complex. Excitatory and inhibitory forces operate in most brain circuits, (a previous post described the stimulation and pruning of brain circuits), and the back and forth regulation between brain and immune.
Immune responses are extremely complex because they are triggered both by the brain’s reaction to external and internal threats, social situations, as well as internal processes such as cellular damage, microbe invasion and cancer. The back and forth communication of brain and immunity make all of these responses much more complex. Excitatory and inhibitory forces operate in most brain circuits, (a previous post described the stimulation and pruning of brain circuits), and the back and forth regulation between brain and immune.
Interactive regulation by both brain and immune allows the detailed responses to bacteria, virus, damaged cells, and cancer, while at the same time, responding to the world at large and response to illness. Infection may be important, but response to other dangers might be more important and the brain has to be able to judge and interact with the immune system.
If fight and flight takes precedence over inflammation for a time, then the brain will decrease the immune activity. If the inflammation is more serious, immune will increase inflammation activity, possibly causing collateral damage. Viral infections occur through contact, and the individual may need to isolate. When exhausted, when extremely fearful, starved, traumatized the individual may need to decrease inflammation for a time to escape.
What are the mechanisms for this complex interaction of the brain and immunity?
Immune and Brain Regulation of Genes
 Microbes in the body trigger two sets of genes: a set of genes that produce inflammation and set that attempts to control the effects of the inflammation. Inflammation is a very complex multi layered process, which can not only kill invaders but also hurt the body’s organs.
Microbes in the body trigger two sets of genes: a set of genes that produce inflammation and set that attempts to control the effects of the inflammation. Inflammation is a very complex multi layered process, which can not only kill invaders but also hurt the body’s organs.
The first response to bacteria triggers cytokines including interleukin-1beta (IL1B), Interleukin-6 and tumor necrosis factor (TNF). These signals stimulate a nuclear factor, which starts DNA transcribing new proteins – called nuclear factor –kB (NF-kappaB) and a specific protein that called activator protein (AP1).
 Viruses stimulate a different response. This response involves totally different signals, namely, interferon type I, (IFN) and use a transcription factor called interferon regulating factor, (IRF).
Viruses stimulate a different response. This response involves totally different signals, namely, interferon type I, (IFN) and use a transcription factor called interferon regulating factor, (IRF).
Both of these responses can be very strong and can produce further illness such as autoimmune disease where immune cells attack normal cells, including creating sepsis where infection and inflammation spread throughout the blood stream. Cardiac disease, diabetes and brain damage also increase through inflammation.
To avoid these serious consequences there are several pathways that attempt to regulate the severity of the immune response. These pathways affect the same sets of genes. When cells are dying, this second level response is triggered.
Critical Response to Control Inflammation Effects via Brain
 The brain attempts to understand the level of danger and the physiological responses occurring. It then signals to regulate the immune processes. The brain uses hormones and neurotransmitters for this purpose.
The brain attempts to understand the level of danger and the physiological responses occurring. It then signals to regulate the immune processes. The brain uses hormones and neurotransmitters for this purpose.
The brain, consciously and unconsciously, attempts to understand circumstances in both the external physical and social environment. The brain, also, monitors the internal bodily environment and makes adjustment to respond to external threats (see post on body maps).
When the brain senses a predator, the fight or flight response is triggered—activating adrenaline, adrenal steroids and many neurotransmitters from the sympathetic nervous system. Another response of external threat is extreme cold or heat, where the body alters the temperature.
 These responses affect every cell in the body, importantly, the immune system. When the inflammation has become too extreme, or when the body needs to use energy for a more important action, then a variety of cytokines and steroids are secreted to lower the inflammation and antiviral response.
These responses affect every cell in the body, importantly, the immune system. When the inflammation has become too extreme, or when the body needs to use energy for a more important action, then a variety of cytokines and steroids are secreted to lower the inflammation and antiviral response.
Steroids, secreted by the adrenal, have a major effect on stopping inflammation, the response to bacteria and trauma. It is well known that steroids are used as medication for inflammation of all types. But, it is less well known that they also control the response to viruses as well.
Steroids are secreted in the adrenal gland after being stimulated by a brain circuit from the hypothalamus and pituitary, called the hypothalamic-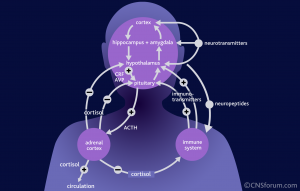 pituitary-adrenal axis (HPA axis). The previous post on brain and immune dual function molecules discussed steroid receptors in all immune cells. Being lipid soluble, steroids can go through the membranes to bind to these receptors inside the cell (like some vitamins, such as D). Stimulating the steroid receptor signals to genes in the nucleus of the cells to stop the inflammation process. One mechanism triggers new genes such as the NFKBIA gene, as well as counteracting NF-kappaB and AP1.
pituitary-adrenal axis (HPA axis). The previous post on brain and immune dual function molecules discussed steroid receptors in all immune cells. Being lipid soluble, steroids can go through the membranes to bind to these receptors inside the cell (like some vitamins, such as D). Stimulating the steroid receptor signals to genes in the nucleus of the cells to stop the inflammation process. One mechanism triggers new genes such as the NFKBIA gene, as well as counteracting NF-kappaB and AP1.
Under stress, the brain also stimulates signaling pathways affecting widespread genes that regulate internal physiology, affecting all bodily organs.
Steroids are now known to be, perhaps, the most critical factor in controlling inflammation.
Sympathetic Nervous System Critical in Regulating Inflammation
Previously it was discussed that immune cells respond through a wide variety of receptors, including special immune receptors, vitamin receptors, and those for neurotransmitters. (post Dual function Molecules of Brain and Immunity). The sympathetic nervous system (and the complementary parasympathetic nervous system) send nerves to most of the body, and have major effects on the critical organs such as heart, lungs, GI, muscles, etc.
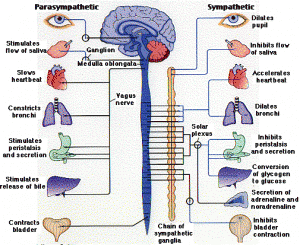 It is important to note that sympathetic nerves travel to all blood vessels (and the space around the blood vessels) as well as all lymph tissues (lymphatic vessels, lymph nodes, and the brain of immunity—the thymus). Norepinephrine is secreted from the sympathetic nervous system into all of these tissues where immune cells operate and it has a major effect in altering the genes inside lymphocytes, the most important immune cells (antibody producing B lymphocytes and T cells including the vital CD4 and CD8).
It is important to note that sympathetic nerves travel to all blood vessels (and the space around the blood vessels) as well as all lymph tissues (lymphatic vessels, lymph nodes, and the brain of immunity—the thymus). Norepinephrine is secreted from the sympathetic nervous system into all of these tissues where immune cells operate and it has a major effect in altering the genes inside lymphocytes, the most important immune cells (antibody producing B lymphocytes and T cells including the vital CD4 and CD8).
Norepinephrine receptors on lymphocytes trigger several complex cascades affecting the innate and adaptive immune systems. Innate T helper cells trigger genes to make cytokines, IL4 and IL5, and decrease IFN and IL12B. Also, it increases new stem cells from the blood—precursors of natural killer cells, neutrophils and monocytes.
In adaptive immunity norepinephrine responses suppress interferon against viruses and increase cytokines, IL1B, IL6 and TNF that promote inflammation.
Social Stress Effects Meditated by Brain through Immune
Another effect of the nervous system to stress includes stimulating new cytokines (signaling molecules like neurotransmitters).
Acute social stress increases IL6, IL1B in the blood and activity of NFkB in immune cells. This also increases white blood cell production of cytokines–lipopolysaccharide, LPS, from microbes stimulating toll like receptors, an important receptor for immune stimulation of inflammation. There is greater effect of the sympathetic system on IL6 in women and this may explain why women have much more inflammation and autoimmune disease.
Steroid Effect in Long Lasting Stress Is Different
 Long lasting stress stimulates genes that increase inflammation with or without steroid increase. It appears that many genes in this situation reduce the factors that stimulate steroids–producing increased blood steroids, while there is suppression of the genes that make steroids. This is based upon a change in the steroid receptors inside of cells. Instead of making more steroids, genes make more NFkB and AP1 that increases inflammation. This complex process of reducing the effectiveness of the steroid receptor occurs in both animals and humans who have prolonged stress.
Long lasting stress stimulates genes that increase inflammation with or without steroid increase. It appears that many genes in this situation reduce the factors that stimulate steroids–producing increased blood steroids, while there is suppression of the genes that make steroids. This is based upon a change in the steroid receptors inside of cells. Instead of making more steroids, genes make more NFkB and AP1 that increases inflammation. This complex process of reducing the effectiveness of the steroid receptor occurs in both animals and humans who have prolonged stress.
Sleep Effects
 Another factor in regulating inflammation appears to be sleep. With little sleep, cytokines increase inflammation by stimulating the transcription factor NFkB. Night workers, experimental sleep deprivation and others with little sleep have increased markers of inflammation including CRP, c-reactive protein.
Another factor in regulating inflammation appears to be sleep. With little sleep, cytokines increase inflammation by stimulating the transcription factor NFkB. Night workers, experimental sleep deprivation and others with little sleep have increased markers of inflammation including CRP, c-reactive protein.
Cytokines and interferon have major effects on sleep, both of which alter dreaming, or non REM sleep, NREM. IL6 and interferon decrease deep sleep with increase dreaming. In animals TNF increase NREM and decrease REM. There is also a higher mortality with extremely high REM. In general there is activation of many immune factors in sleep.
Fatigue is stimulated by immune response. Inflammation increases fatigue, which is a major problem in chronic inflammation diseases, such as multiple sclerosis, arthritis, and cancer. Cancer often activates NFkB, increasing inflammation. After cancer treatment there are often lengthy periods of fatigue, which is associated with increased IL1B, IL6 and TNF. Medications that counteract TNF have been helpful, which would imply that cytokines are significant in this fatigue.
Neurotransmitter Effects
The signals to start inflammation and antivirus processes are transmitted from body organs to the brain with alterations in many different neurotransmitters. The cytokines that are first triggered when inflammation begins also change neurotransmitters—decreasing serotonin, norepinephrine, and dopamine, all critical factors in modulation of human behavior.
Sickness Behavior Mediated by Brain to Help Immune Response
 In a previous post, the complex process that triggers the sickness feeling was introduced. Brain endothelial cells that are responding to inflammation in the body secrete prostaglandin E2 causing fever and metabolic changes.
In a previous post, the complex process that triggers the sickness feeling was introduced. Brain endothelial cells that are responding to inflammation in the body secrete prostaglandin E2 causing fever and metabolic changes.
Emotional changes, lack of energy, and unhappiness caused by infection have complex immune and brain pathways. Different cytokines appear to stimulate different behavior syndromes in the brain.
Cells in the hypothalamus and hippocampus secrete IL1. Several critical brain regions related to emotion have decreased signaling because of cytokines—including decreased signaling between the subgenual anterior cingulate cortex, the amygdala, and the prefrontal cortex. The center of reward in the brain, the nucleus accumbens, has reduced activity and therefore there is little enjoyment—a state called anhedonia that occurs with depression, grief, and with infection.
Many brain regions are affected by immune secreted cytokines. One region can be triggered, which then affects communication with other major hubs. At least the following regions are involved in these feedback loops of immune response:
- Hypothalamus, the major center of physiological regulation including sleep, metabolism and eating
- The amygdala, the center of social emotion, social interaction, fear and emotional memory
- The hippocampus, the center of learning and short-term memory
- The anterior cingulate cortex, described in previous posts as a primary place where integration of emotion, physiological responses, body maps, and learning occur
The sickness feelings, first stimulated by cytokines, but, then regulated by the brain, also includes alterations of the energy resource allocation in the body. Energy increases the immune activity, decreases muscular activity, reducing contact with others to avoid spreading infection, and increasing the temperature to kill microbes.
There are multiple pathways for the inflammation signals to alter activity in the brain. Cytokine signals in the blood stimulate receptors near the brain ventricles. The blood vessels in the brain start to produce signals that produce more cytokine signals inside the brain itself. A process has been discovered that special carrier molecules actually transport cytokines across the blood brain barrier. Sensory nerves near the inflammation site also send signals.
Mental Events and Activity Cause Positive and Negative Immune Effects
Meditation and community service have positive immune effects and depression and stress have negative effects (see post on effects of meditation). Interferon used in the treatment of cancer can trigger depression, including lack of sleep, lack of interest and fatigue. TNF can reverse of these symptoms and is being evaluated for some depression.
Earlier it was mentioned that activities stimulating enjoyment through service or charity have a dramatic immune effect, while other ordinary personal pleasures do not. Specifically there were many changes in genes of immune cells and cytokines. With charitable giving there was a decrease in the genes activities of pro-inflammatory cytokines such as IL1B, IL6, IL8, and TNF. There was increased expression of genes involved in type I IFN antiviral responses including IFI-, OAS-, and MX- family genes. There was also increased IgG1 antibody synthesis.
 Meditation, eating certain foods, and exercise can, also, reduce inflammation. Psychological therapies have been shown to decrease cytokines that increase inflammation.
Meditation, eating certain foods, and exercise can, also, reduce inflammation. Psychological therapies have been shown to decrease cytokines that increase inflammation.
Meditation has dramatic effects on immunity. Recent studies show decreased immune inflammatory factors interleukin 6, and NF-KappaB, and an increase in the important antiviral factor IRF1. Other studies showed decreased inflammation with local skin burns, fewer colds and decreased stress hormones.
Long term meditators and novices both showed epigenetic gene expression changes, with greater changes for the long-term meditators. The specific genetic changes appeared to make mitochondria more resilient. The genes that changed involved increases in energy metabolism, mitochondrial function, insulin secretion, telomere maintenance and decrease in inflammation and oxidative stress responses. Specifically, mitochondrial ATP synthase and insulin (INS) were upregulated, and NF-kB pathway genes were down regulated. Other studies show that with Tai Chi there were reduced blood levels of IL-6 in older adults; and reduction in respiratory infections with meditators (better with both exercise and meditation). With Kriya Meditation by dementia caregivers there were 68 gene changes related to decreased inflammation.
A study of yoga by dementia caregivers reversed NF-kappaB and IRF in genes of blood cells. Genes were increased for immunoglobulin and decreased pro inflammatory cytokines. There was, also, alterations in dendritic immune cells and B lymphocytes including reduced NF-kappaB and increased antiviral IRF1.
A study of a mindfulness retreat showed decrease in cortisol, inflammation, and inflammation markers.
Brain and Immunity Fight Internal and External Foes Together
 Brain and immunity work as one for defense against bacteria and virus, response to dangerous situations including social interactions, and the effects of positive and negative mental activity. Human activity such as meditation and the pleasurable acts of service and charity had major positive effects on immune function. Conversely, inflammation creates a wide variety of mental effects such as sickness feelings, lack of interest and fatigue. In both the small realms of cells as well as the larger realms of human interaction, the immune and brain systems respond to all internal and external experiences together.
Brain and immunity work as one for defense against bacteria and virus, response to dangerous situations including social interactions, and the effects of positive and negative mental activity. Human activity such as meditation and the pleasurable acts of service and charity had major positive effects on immune function. Conversely, inflammation creates a wide variety of mental effects such as sickness feelings, lack of interest and fatigue. In both the small realms of cells as well as the larger realms of human interaction, the immune and brain systems respond to all internal and external experiences together.
It has been shown that mental events stimulate complex molecular changes in wide ranging circuits in the brain, called neuroplasticity. These changes involve sudden changes in gene networks that manufacture many new complex protein machines to function in the nerves. These mental events also influence the immune cells that have an equally complex response through unique genetic changes in immune cells and complex communication among a wide variety of cells and brain circuits. Both of these responses to mental events include thousands of signals from cytokines and neurotransmitters.
Once again, a remarkable influence of thought and action are observed on the complex molecular processes inside the nucleus of cells as well as larger networks of cells in both the brain and the immune system.