 Many recent developments point towards the immune and nervous systems being the same system, including use of the same critical molecules and signaling pathways. Two recent developments in particular show the intimate connection—the origin of synesthesia and destruction of synapses in Alzheimer’s disease. The previous dogma that the CNS is “immune privileged” now appears overly simplistic as vast networks of immune molecules are found in the CNS. Also, previous posts have shown how immune cells are signaled and controlled by neurons and respond to neurotransmitters. This post will highlight new information on synesthesia, Alzheimer’s, and especially the vast complex signaling of the MHC, the immune systems major histocompatibility complex, that determines whether our cells are considered “self” or are destroyed as an “other”, such as a microbe or cancer cell. This compatibility complex of genes and proteins, the backbone of all immunity, uses dual function molecules for brain and immunity.
Many recent developments point towards the immune and nervous systems being the same system, including use of the same critical molecules and signaling pathways. Two recent developments in particular show the intimate connection—the origin of synesthesia and destruction of synapses in Alzheimer’s disease. The previous dogma that the CNS is “immune privileged” now appears overly simplistic as vast networks of immune molecules are found in the CNS. Also, previous posts have shown how immune cells are signaled and controlled by neurons and respond to neurotransmitters. This post will highlight new information on synesthesia, Alzheimer’s, and especially the vast complex signaling of the MHC, the immune systems major histocompatibility complex, that determines whether our cells are considered “self” or are destroyed as an “other”, such as a microbe or cancer cell. This compatibility complex of genes and proteins, the backbone of all immunity, uses dual function molecules for brain and immunity.
Alzheimer’s and Immune Receptor
Recent research shows that immune proteins on the surface of neurons near synapses are receptors for beta amyloid, the peptides that form plaques in Alzheimer’s related to the destruction of synapses and neurons. In fact, this study showed that the immune protein receptor, PirB, by attracting beta amyloid is a mechanism that starts the amyloid cluster on the neuron, tripping a cascade of activity that kills brain cells and causes Alzheimer’s dementia.
Synesthesia Hyper Connectivity and Immune Pruning
 Another set of research shows that synesthesia might well be related to immune regulation of the nervous system. While synesthesia is associated with increased creativity and cognition, there are also associated medical issues. It is largely hereditary but also occurs after injury. Recently, several genes have been shown to be important in synesthesia and have critical functions in the immune system (2a24, 5q33, 6p12, and 12p12). These gene regions are highly connected to interleukin 17, as well as many other immune molecules. In addition, there is a possibility that synesthetes have an increased incidence of multiple sclerosis and autism, both linked to immune disorders and unusual brain connectivity. Also, with the sudden appearance of synesthesia after brain injury, immune problems could be the cause. Another study showed that there is an association with irritable bowel and synesthesia.
Another set of research shows that synesthesia might well be related to immune regulation of the nervous system. While synesthesia is associated with increased creativity and cognition, there are also associated medical issues. It is largely hereditary but also occurs after injury. Recently, several genes have been shown to be important in synesthesia and have critical functions in the immune system (2a24, 5q33, 6p12, and 12p12). These gene regions are highly connected to interleukin 17, as well as many other immune molecules. In addition, there is a possibility that synesthetes have an increased incidence of multiple sclerosis and autism, both linked to immune disorders and unusual brain connectivity. Also, with the sudden appearance of synesthesia after brain injury, immune problems could be the cause. Another study showed that there is an association with irritable bowel and synesthesia.
Recent research shows that much of the brain is multisensory, that is, (unlike the old module theory of the brain) most of the brain’s neurons have multiple connections to other senses and brain regions. Synesthesia, the unusual combinations of senses such as shape, color, smell and taste, is now known to be much more common than previously thought.
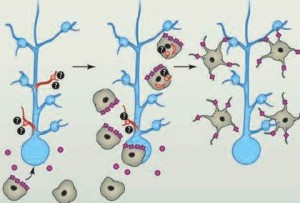 It is now being questioned whether immune pruning of synapses causes the hyper connectivity in synesthesia. Structural differences have been observed in synesthesia with increased connections in the cortex. The excess connectivity allows one area, such as vision, to directly activate another region, auditory. Other research points to decreased feedback of the parietal cortex not suppressing information from lower regions (excessive excitatory neurons, less inhibitory). Probably, both of these occur because less inhibition would also foster increased connectivity.
It is now being questioned whether immune pruning of synapses causes the hyper connectivity in synesthesia. Structural differences have been observed in synesthesia with increased connections in the cortex. The excess connectivity allows one area, such as vision, to directly activate another region, auditory. Other research points to decreased feedback of the parietal cortex not suppressing information from lower regions (excessive excitatory neurons, less inhibitory). Probably, both of these occur because less inhibition would also foster increased connectivity.
One theory of synesthesia is that during fetal pruning (of 9/10 of the trillion neurons) some of the connections between sensory brain regions are not pruned. There is much to learn about the pruning process that is immense just after birth, and continues at a much smaller level every night in adulthood. This increased connectivity can occur from changes in immune function.
Immune and Nervous Systems Are One
There is extensive evidence of the connections between the immune system and the nervous system. This has been addressed in two of my previous posts (Neurons and Immune Cells Working Together, and “Wired and Wireless Components of the Brain” in Scientific American Guest Blog) where it is shown that the nervous system is highly involved in all aspects of inflammation and is constantly sending information that increases the immune cell’s response. In fact, immune cells like lymphocytes respond to neurotransmitters like dopamine in this process. On the other side there is constant surveillance and regulation of the brain by immune cells.
 But, what has not been appreciated until recently is that many signals, pattern recognition receptors, and a large number of proteins have a dual function in both the immune and nervous systems. When the immune system prunes synapses it is critical in the development of the brain. The signaling in the immune cells is at least, if not more, complex than in the brain, and often uses the same signaling pathways.
But, what has not been appreciated until recently is that many signals, pattern recognition receptors, and a large number of proteins have a dual function in both the immune and nervous systems. When the immune system prunes synapses it is critical in the development of the brain. The signaling in the immune cells is at least, if not more, complex than in the brain, and often uses the same signaling pathways.
Many genes have been shown to produce proteins that aid the development of both the cerebral cortex and immune function. These genes in brain cells influence the travel and guidance of axons as they grow, and the production and pruning of synapses. The immune system has direct influence on much of this activity as well, possibly through actions of the microglia (astrocytes also have major activity is producing and maintaining synapses – see post). The immune system is, also, critical in the development and neuroplasticity of the excitatory glutamate neurons and synapses.
Old Dogma of Nervous System and Immune
The previous dogma has been that the brain is “immune privileged” (very little immune activity) because there aren’t many typical immune cells, such as macrophages, dendritic cells, and T and B lymphocytes. There are, of course, microglia that are a type of brain macrophage. But, recently, this view is being altered because of the enormous amount of specific immune molecules with vital properties for brain function, many of them on the surfaces of brain cells. These thousands of molecules have a dual function in the immune and nervous systems. This includes cytokines, complement and the MHC proteins.
This post will describe the three major areas of dual functions that are being found, but with emphasis on the MHC, one of the most important factors in the immune system.
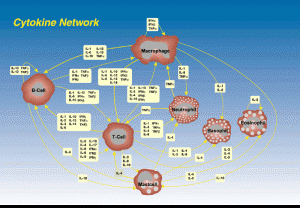
Cytokines
The Cytokine system of signals is now known to be as complex as the neurotransmitter systems. These signals regulate the critical movement of T cells, guiding them to travel to the various lymph nodes and the thymus (a brain of the immune system), in order to meet the cells that present antigens that they will then chase. Cytokines are critical to the movement of all immune cells in the process of fighting bacteria including during inflammation. Cytokines are critical signals for all aspects of immune function, and for signaling from immune cells to brain cells.
Now, they also have been shown to have a dual function in that they are involved in stimulating new brain cells and increasing neuroplasticity. The signaling with cytokines occurs from both brain cells and immune cells.
Complement System
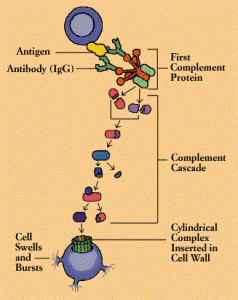 The critical family of complex proteins called the complement system has been shown to have dual functions in immune and nervous systems. This complex cascade of molecules tags synapses for pruning as well as microbes, defective cells, and cancer cells for elimination. Like MHC these processes appear to limit growth of the CNS—that is, they limit connectivity.
The critical family of complex proteins called the complement system has been shown to have dual functions in immune and nervous systems. This complex cascade of molecules tags synapses for pruning as well as microbes, defective cells, and cancer cells for elimination. Like MHC these processes appear to limit growth of the CNS—that is, they limit connectivity.
Most of the actions of both the complement system and the MHC are to control brain activity through inhibition. Without this constant regulation, the brain activity would spiral out of control (which it does in some illnesses).
Immune MHC
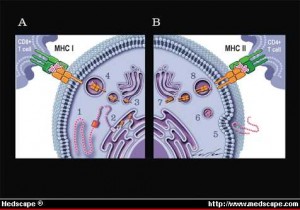 The major histocompatibility complex, MHC, is the critical set of genes, proteins, and receptors that not only distinguishes what are our own cells, “self” cells, (our own cells that should not be killed along with the intruders), and “alien” cells that need to be destroyed. Pieces of proteins from inside of cells combined with MHC proteins are placed on the surface of almost all of our cells and are critical to the adaptive immune systems processes of fighting microbes and cancer. Placing MHC with specific peptides from intracellular proteins on the surface of cells produces a neon light signal for T cells to understand what is occurring inside the cells. This tells the T cell to leave the cell alone (our ordinary non infected self cells) or to attack (an infected, defective or cancerous cell). These many MHC signs on the outside membrane allows immune T cells to determine whether a cell has normal material or abnormal material inside. When abnormality is found, immune response and destruction of the cell occurs.
The major histocompatibility complex, MHC, is the critical set of genes, proteins, and receptors that not only distinguishes what are our own cells, “self” cells, (our own cells that should not be killed along with the intruders), and “alien” cells that need to be destroyed. Pieces of proteins from inside of cells combined with MHC proteins are placed on the surface of almost all of our cells and are critical to the adaptive immune systems processes of fighting microbes and cancer. Placing MHC with specific peptides from intracellular proteins on the surface of cells produces a neon light signal for T cells to understand what is occurring inside the cells. This tells the T cell to leave the cell alone (our ordinary non infected self cells) or to attack (an infected, defective or cancerous cell). These many MHC signs on the outside membrane allows immune T cells to determine whether a cell has normal material or abnormal material inside. When abnormality is found, immune response and destruction of the cell occurs.
MHC On Brain Cells
It is important to note that the MHC is also on the surface of almost all of the CNS cells as well. Of the MHC classes I and II, the MHC class I proteins, called MHCI, are involved in brain development, as well as neuroplasticity and synapse production in adults. The MHC is one of the largest and most significant sets of genes in humans and is critical in autoimmune diseases like multiple sclerosis, irritable bowel, and arthritis.
 There are competing, stimulating and inhibitory, processes in the creation of synapses and their pruning. The connectivity of the brain is determined by the travelling axons meeting dendrites and forming synapses. While it is not completely understood, it does appear that the immune system, especially the MHC (also the complement and cytokine systems) regulate this process along with the neural factors, like BDNF, that stimulate neuroplasticity. Much of this research has been proven in the visual system, but also throughout the other parts of the brain.
There are competing, stimulating and inhibitory, processes in the creation of synapses and their pruning. The connectivity of the brain is determined by the travelling axons meeting dendrites and forming synapses. While it is not completely understood, it does appear that the immune system, especially the MHC (also the complement and cytokine systems) regulate this process along with the neural factors, like BDNF, that stimulate neuroplasticity. Much of this research has been proven in the visual system, but also throughout the other parts of the brain.
A balance of two sets of proteins—with functions of promoting or inhibiting synapses—determines connections in the brain. Many of the inhibitory molecules are immune proteins. The MHCI appears to regulate the outgrowths of the cell, neurites, travel guidance and connections involved in synapses, and the neuroplasticity in the visual system.
There is a vast literature of the critical functions of the MHCI in all aspects of immunity. But, just recently the MHCI have been shown to be critical in brain development, neuroplasticity, and repairing nerves in the peripheral nervous system.
MHCI Proteins on CNS Cells
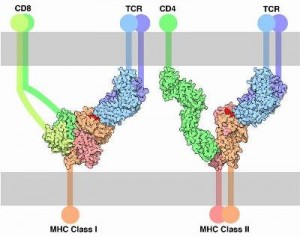 MHCI molecules are found in the CNS on neurons and glial cells. The greatest amount is just after birth. MHCI was at first thought not to be on neurons, but now they are seen on axons and dendrites, at pre and postsynaptic neurons. MHCI mRNA is found in dendrites of hippocampus cells. MHCI have been found on cultured astrocytes and activated microglia. The MHCI expression is altered when cells are activated.
MHCI molecules are found in the CNS on neurons and glial cells. The greatest amount is just after birth. MHCI was at first thought not to be on neurons, but now they are seen on axons and dendrites, at pre and postsynaptic neurons. MHCI mRNA is found in dendrites of hippocampus cells. MHCI have been found on cultured astrocytes and activated microglia. The MHCI expression is altered when cells are activated.
Also, MHCI receptors are found throughout the CNS. Two important receptors in this process are PirA, activating, and PirB, inhibiting, which have recently been shown to attract amyloid particles and create new amyloid plaques (a hallmark of Alzheimer’s disease — either a cause or an effect). PirB, is found in hippocampal neurons, in the axon growth cones and at synapses. It clearly affects brain development. This same receptor in the immune system signals for Natural Killer cells and suppresses synapse formation. There are many other very complex immune receptors and signaling pathways now found to regulate neuroplasticity. There is now substantial evidence that MHCI mediates the pruning of connections in the visual systems.
- Injury and Strokes: MHCI and PirB limit axons after injury and increases the damage. After strokes there is more MHCI and PirB. In the periphery at times MHCI does the opposite and it appears to maintain sciatic synapses after injury as opposed to the opposite effect in the CNS.
- MHCI levels on neurons are regulated by cytokines. In degenerative disorders there are altered levels of cytokines the blood and CSF.
- While it is not as well known how MHCI and signaling receptors operate in astrocytes and microglia, it is possible that MHCI is involved in the critical microglia function of synaptic pruning.
- As well as the visual system, there is now evidence for MHCI regulating development of the cerebellum and the olfactory system. In the cerebellum it appears to regulate motor learning and synaptic plasticity. MHCI also appears critical in the guidance of axons as they travel to their sites of synapse. MHCI is shown to limit growth of some axons, and dendrites. MHCI antibodies increase and Ly40 decrease levels of molecule synapsin.
MHCI Inhibits Differently In Different Brain Regions
 MHCI inhibits initial CNS connections. Without MHCI there are too many connections in the developing visual system. Many areas of research point to the fact that MHCI restricts synapse density in the visual system and hippocampus. It is not clear whether this process is limiting of synapses, or increasing pruning.
MHCI inhibits initial CNS connections. Without MHCI there are too many connections in the developing visual system. Many areas of research point to the fact that MHCI restricts synapse density in the visual system and hippocampus. It is not clear whether this process is limiting of synapses, or increasing pruning.
MHCI affects transmission differently in different brain regions. Levels of MHCI molecules affect the balance of excitation and inhibition in the cortex. They are shown to limit NMDA function and the increase of AMPAR that occurs in neuroplasticity (see post on Glutamate Neuroplasticity). MHCI have a dual role of both regulating initial synapse creation and strengthening the synapse later.
MHCI Connects with Many Other Important Proteins for Signaling
MHCI represents yet another vast regulatory network responding instantly to thought and circumstances in neuroplasticity.
 MHCI functions through binding to other proteins that then stimulate pathways. These complex pathways are mediated though the range of immune proteins and receptors that exist in and on neurons. MHCI binds to insulin factors and receptors, interleukin2, intercellular adhesion molecule and the epidermal growth factor. MHCI is present on axons, growth cones, dendrites, and synapses.
MHCI functions through binding to other proteins that then stimulate pathways. These complex pathways are mediated though the range of immune proteins and receptors that exist in and on neurons. MHCI binds to insulin factors and receptors, interleukin2, intercellular adhesion molecule and the epidermal growth factor. MHCI is present on axons, growth cones, dendrites, and synapses.
Because of the great complexity of the MHCI molecules there are many different receptors and signaling pathways. It appears that MHCI alters the receptors, signals and pathways based upon the stage of development (fetus, young or adult), the specific environmental stimulus, the different types of brain cells, and the different regions.
Dual Function Molecules for Brain and Immunity
Despite the physical barriers between the immune system with lymphatics, spleen, and bone marrow; and the nervous system of brain circuits, astrocytes, axons and dendrites, there is a constant interaction in all functions of the immune system and nervous system. All of the major signaling molecules of the immune system — 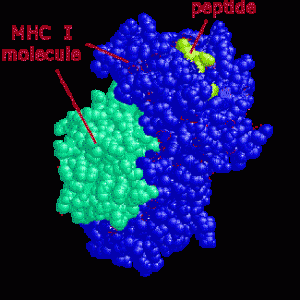 complement, cytokines, chemokines, and MHC — have dual roles in the nervous and immune systems. The immune regulates the functions of the brain, and the nerves do the same for every aspect of immunity. The vast complexity of the molecular signaling in neurons is only equaled by the remarkable complexity of immune signaling. Many of the signaling pathways use similar enzymes. In fact, there is no real way to draw a distinction between the nervous and immune systems.
complement, cytokines, chemokines, and MHC — have dual roles in the nervous and immune systems. The immune regulates the functions of the brain, and the nerves do the same for every aspect of immunity. The vast complexity of the molecular signaling in neurons is only equaled by the remarkable complexity of immune signaling. Many of the signaling pathways use similar enzymes. In fact, there is no real way to draw a distinction between the nervous and immune systems.
Mind instantly alters the complex molecules in wide circuits throughout the brain by neuroplasticity (see post, Neuroplasticity Update). Research into meditation (see post Meditation Update 2013) shows that immune circuits are also stimulated through mind. Future posts will further elaborate on the intimate relationship of the brain, the mind and immunity.