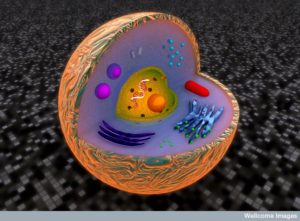
 Vacuoles are unique intracellular vesicles with many different functions. Some store critical molecules. Some store water or fat. Others are factories of destruction for waste, debris and mis folded proteins. Previous posts have described how individual organelles, like mitochondria have independent intelligent activity. Some vacuoles, also, provide critical independent activity for the life of a cell.
Vacuoles are unique intracellular vesicles with many different functions. Some store critical molecules. Some store water or fat. Others are factories of destruction for waste, debris and mis folded proteins. Previous posts have described how individual organelles, like mitochondria have independent intelligent activity. Some vacuoles, also, provide critical independent activity for the life of a cell.
It is odd that some microbes find vacuoles a wonderful place to live and have adapted to this particular niche. In some cases, symbiotic microbes make vacuoles their permanent home. Since it is a place used by the cells to isolate and then remove or kill microbes, the visitors must use great ingenuity to be able to operate from this location. Using complex systems of sensing and attack, clever cells and microbes fight over vacuoles.
Previous posts describe the many complex ways that cells and microbes combat each other including devising specific proteins and RNAs. It is a vital battleground for survival of both the cell and the microbe.
Many Kinds of Vacuoles
 Membrane-surrounded organelles are different in plants, animals and fungi. They are a warehouse of small molecules for the cell. They isolate materials that threaten the cell such as waste products. In plants they can be quite large, holding large amounts of water and other materials supporting the structure of flowers and leaves. Another specialized plant vacuole holds proteins in seeds needed for germination. Vacuoles maintain pH and hydrostatic pressure and are involved in the process of removing dangerous substances.
Membrane-surrounded organelles are different in plants, animals and fungi. They are a warehouse of small molecules for the cell. They isolate materials that threaten the cell such as waste products. In plants they can be quite large, holding large amounts of water and other materials supporting the structure of flowers and leaves. Another specialized plant vacuole holds proteins in seeds needed for germination. Vacuoles maintain pH and hydrostatic pressure and are involved in the process of removing dangerous substances.
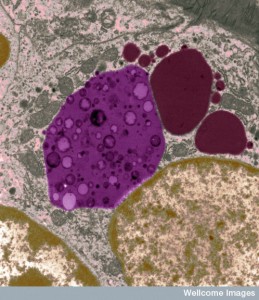
In mammals they can be much smaller. In humans, an important type is called a lysosome, which is a critical part of the defense of the cell. Lysosomes are a factory of destruction with enzymes to break down mis folded proteins and microbes. They are part of the secretory pathways that make membrane and they fuse with other vesicles in multiple complex cellular processes for the maintenance of the cell. The post on the essential metabolic protein mTOR showed the unique part lysosomes play in maintaining the metabolic milieu of the cell.
Vacuoles are an important part of autophagy, which is a critical process in every cell for maintaining a balance between building up the cell and breaking it down. In the extreme, the process kills cells that are determined to not be fixable often because of microbe invasion. Autophagy is pat of the pathways that either produce or degrade critical molecules used by the cell.
Microbes At Home in Vacuoles
Microbes are able customize their own vacuole homes. The cell, however, in its constant battle with microbe invasions, has devised many ways to find and expose the microbes living in vacuoles. It is a battle of intelligent behaviors.
When a microbe invades the cell, there are many possible outcomes based on the maneuvers of the much smaller microbe to thwart the attacks from the cell’s immune systems. There are many barriers to microbe movements in the cell, both chemical and physical barriers and the attacks from the immune system.
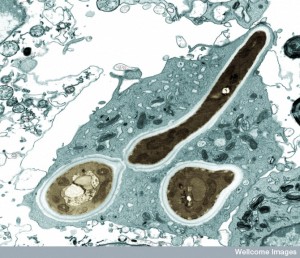
Specific microbes, bacteria and protozoa, can live and reproduce in the vacuole. Called “professional microbes” by some, they live their entire lives inside of the vacuole. Some only stay for a time and use it as a base of operations to leave and attack the cell. The microbe is able to produce secreted molecules that alter the living accommodations in the vacuole. Sometimes, by customizing their living conditions, they inadvertently send signals to the cell about where they are hiding.
There are a wide variety of different microbes using different techniques for hiding. Cells develop many different counter measures as well. Cells have many different methods of sensing the existence of microbes hiding in vacuoles.
The first involves the cell sensing specific molecules that are associated with the microbe. Cells have many different compartments each with a unique environment. The vacuole has complex secretion mechanisms that can release some of the microbe’s molecules, warning the cellular defenses. This can occur by species specific lysosome molecules used to destroy the microbe. The microbe will secrete counteracting molecules that can, also, be released.
Warning Signals For the Cell
From Adenosine
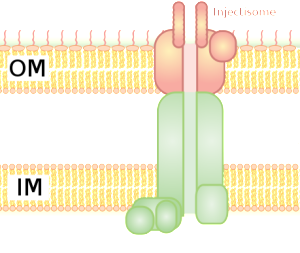
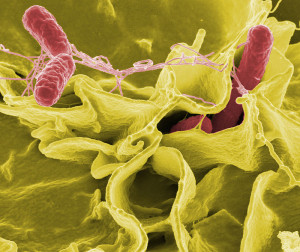
To facilitate entry into the vacuole, the microbe alters the vacuole’s membrane and then punctures it with a secretion system. There a variety of secretion systems that can appear like syringes or phage viruses. S. Typhimurium uses type III secretion system (T3SS). The cell’s reaction to this is to build a complex immune structure called the inflammasome made of many large proteins. It is triggered by special receptors and specific cytokines such as IL-1β. The inflammasome can decide to kill the cell to get rid of the microbe hiding in the vacuole.

Another sensor responds to the microbe’s flagellum and another to the secretory system itself. Tuberculosis uses a type 7 secretion system (T7SS), which triggers interferon, but doesn’t kill the microbe in the vacuole. It is not clear how, but the T.B microbe’s DNA can end up outside of the vacuole, which brings about a DNA sensor that, also, triggers the interferon cytokine and the immune response that it triggers.
With salmonella, the vacuole transporters on its membrane take pieces of the salmonella through the membrane to the outside where sensors pick it up. The cell can alter the vacuole itself making it more permeable and releasing molecules from the microbe, which triggers sensors. There are many different complex pathways once the sensors are triggered. In one case, the cell isn’t destroyed but the vacuole is and the debris is sent to phagocytes.
Other Cellular Sensing Mechanisms
 The pattern recognition receptors (PPRs) pick up very particular molecular strands that are connected to infections. If they include structures from microbes they are called microbe-associated molecular patterns or MAMPs. This includes parts of the coating of the microbe and genetic material. When particular molecular patterns cause destruction in the cell, they are called damage or danger associated molecular patterns or DAMPs. These include pieces of damaged organelles or cells. Another pattern involves specific processes or environmental conditions created by the microbes, such as leaky membranes.
The pattern recognition receptors (PPRs) pick up very particular molecular strands that are connected to infections. If they include structures from microbes they are called microbe-associated molecular patterns or MAMPs. This includes parts of the coating of the microbe and genetic material. When particular molecular patterns cause destruction in the cell, they are called damage or danger associated molecular patterns or DAMPs. These include pieces of damaged organelles or cells. Another pattern involves specific processes or environmental conditions created by the microbes, such as leaky membranes.
The PPRs are specifically focused on unique compartments of the cell, such as organelles, vacuoles, and particular unique spaces such as the ER or the nucleus. Some of the sensors are on the outside of cellular membranes (Toll-like receptors TLRs) or on vesicle membranes inside the cell. Other sensors pick up molecules in the middle of the cell such as specific proteins that work with DNA.
 Once the sensor is triggered, then pathways lead to genetic networks producing cytokines to fight microbes including NF-KB and interferon. Others signal particular immune cells. Another response of the sensors is to create the large complexes called inflammasomes.
Once the sensor is triggered, then pathways lead to genetic networks producing cytokines to fight microbes including NF-KB and interferon. Others signal particular immune cells. Another response of the sensors is to create the large complexes called inflammasomes.
If the problems are very difficult, then programed cell death becomes the answer, sacrificing the infected cell and the microbe inside for the greater good of the organism. Another response is autophagy, where the compartment, in this case the vacuole is destroyed.
The Microbe’s Vacuole Home
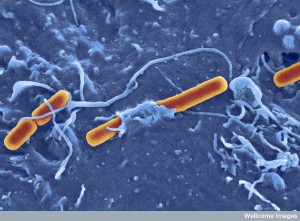 Microbes are quite clever to build a home in the vacuole. First, they have the great difficulty of entering the cell. They must trick the cell into bringing them inside the cell with a vesicle. The cell brings in many messages and material from other cells in this way. (See post on Exosomes and Vesicles.)
Microbes are quite clever to build a home in the vacuole. First, they have the great difficulty of entering the cell. They must trick the cell into bringing them inside the cell with a vesicle. The cell brings in many messages and material from other cells in this way. (See post on Exosomes and Vesicles.)
The microbe directs the process of their own uptake or they can be taken in passively. Microbes use their secretion systems to inject molecules into the cell that alter the scaffolding and the membrane creating avenues to enter the cell. They create an alteration in the membrane that is called ruffling that allows the microbe to enter. Another technique involves the cell’s function of eating debris by surrounding them with a membrane—phagocytosis. Whether active or passively taken in, they are surrounded by a membrane creating a vacuole.
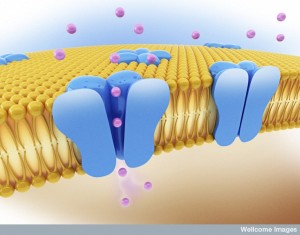 Once inside the cell in their vacuole, the microbe must strengthen the vacuole from attack or manipulation from the cell. Microbes create specific molecules that trigger more scaffolding around the vacuole, by manipulating actin and intermediate filaments.
Once inside the cell in their vacuole, the microbe must strengthen the vacuole from attack or manipulation from the cell. Microbes create specific molecules that trigger more scaffolding around the vacuole, by manipulating actin and intermediate filaments.
The microbes must, also, create food for themselves inside the vacuole. They are somehow able to modify the membrane to allow the diffusion of small molecules for the microbe to eat. They do this by adding porins to the membrane—particular proteins that form channels in the membrane. They are, also, able to change the basic scaffolding of the cell so that vesicles that ordinarily supply nutrients to various compartments of the cell now are sent directly to the vacuole. It is quite remarkable that they can accomplish all of this from inside the vacuole.
The microbes must, also, defend against the attacks on the vacuole. The microbes re model the structure of the vacuole, including membranes, as a defense. They are able to alter the membrane so it is not obvious to the sensors where they are hiding. This protects against the pathways that promote programmed cell death. In the ordinary life of the cell, the vacuole would eventually fuse with other vesicles in the cell and the contents would be either used or destroyed. But, the microbes are able to alter the pH to avoid the destructive lysosomes. In fact, clever microbes are able to completely take over lysosomes as living space. Another technique of Toxoplama gondii alters the vacuole using special proteins so that it cannot fuse and therefore stays out of the ordinary pathways of vesicles in the cell.
Parasitic Microbes Form Their Own Type of Vacuole
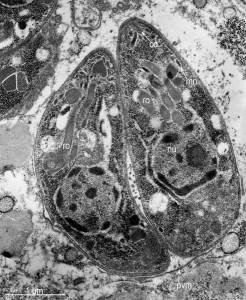
Protozoa produce their own special types of vacuole that ARE different from others, but looks the same under a microscope. Parasitophorous vacuoles, (PVs), allow the parasite to exist and grow within the cell, while protecting it from the host cell defense mechanisms. PVs control the pH, not allowing the vacuole to be acidified by the cell.
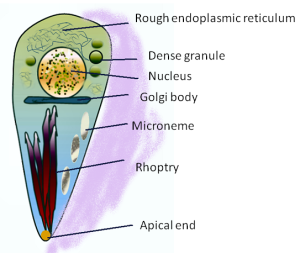
This vacuole is first formed when the microbe enters the cell. It creates the PV with part of the host membrane that surrounds the parasite. Special organelles of the parasite, called the rhoptry and microneme, secrete materials and create the bubble of cytoplasm around the parasite. The microneme binds to red blood cells allowing entry into the cell and the rhoptry secretes proteins to build the vacuolar space.
Protozoa, including malaria Plasmodium and Toxoplasma, have a special apical complex that has unique organelles for secretions using microtubules. These parasites enjoy their double membrane vacuoles by decorating them with special proteins.
Parasites have many very different lifestyles. An extreme version occurs in red blood cells where rapid reproduction can produce 30,000 new merozoites (one form of the protozoan) that then burst the cell, sending them after other cells. Other species reproduce very slowly. Toxoplasma gondii has many varieties. Some trigger sensors that produce interferon that destroys the vacuole, while others do not. Others are able to avoid the acidification of the vacuole, which stops the normal fusion with other organelles in the cell.
Cells Respond to Various Parasites in Different Ways
There are many different ways that the cell can find out that parasites are living inside the cell. They then send a variety of chemicals to alter the vacuole while it is occupied by T. gondii. The cell’s response, also, usually uses cytokines such as interferon.
In many cases, it is not clear how the cell senses the molecules produced by the microbe. Some occur by microbes that die inside the vacuole; some through pores in the membrane produced by the microbes; some are found through vesicles from the vacuole itself that are sent to immune cells directly. Very small vesicles are sent from red blood cells infected by Plasmodium with particles from the microbe. These are eaten by macrophages and then a cytokine response occurs. Exosomes are sent with RNA from hepatitis C RNA virus.
Indirect Sensing
 The microbes secrete molecules when they first enter the cell that allow them to tailor the vacuole environment. But, the cells pick up signals from these molecules. They aren’t picked up by the first innate immune receptors but influence cascades related to the functioning of the immune cells.
The microbes secrete molecules when they first enter the cell that allow them to tailor the vacuole environment. But, the cells pick up signals from these molecules. They aren’t picked up by the first innate immune receptors but influence cascades related to the functioning of the immune cells.
There are many specific pathways whereby microbes’ secretions trigger kinases in the immune signaling cascades. Microbes, also, secrete toxins that inactivate these same pathways and allow the microbe to survive in the cell. The whole range of inflammatory reactions can occur or be blocked by different microbe molecules.
Gondii parasites make multiple different proteins that they place on the membrane of the vacuole and on the outside membrane of the cell itself. Many of these are major modulators of the innate immune response. They are first injected into the cell as they enter the cell to inactivate responses that try to stop their entry. They prevent the usual recognition that would occur at the surface of the vacuole. T. gondii then secretes many others from within the special parasitic vacuole. These trigger false leads for the immune system. They stimulate a response from macrophages to go after milder versions of the parasite, leaving alone the more serious species. There are many variations in the amino acids used that determine which variety it will go after. It is a Trojan horse sending the attacks elsewhere.
Another T. gondii molecule goes to the cell’s nucleus stimulating genetic networks that influence the cell to avoid looking for the parasite in the vacuole. The parasite has developed a large number of different molecules to counteract many of the cell’s attacks. The cell responds to some of these molecules with their own molecules in attack. (see post on the battle of plants and microbes using microRNAs).
Altered Self
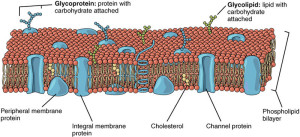
Another method is altering the self identity of the cell. Each cell has ways of identifying its own molecules and sensing when there are new unexplained particles. However, the microbe can utilize the molecules identified as belonging to the “self” of the cell in unusual ways. If the microbe makes a hole in the vacuolar membrane in order to allow entry of nutrients or to secrete its own toxins, the cell can sense that its own molecules are not operating properly.
A particular sensor is galactic, which notices damage to the membrane of a vacuole. Galectins are sugar-based molecules that can bind to particular proteins. They can exist spanning the membrane from inside to the outside; they can be secreted and they can be generally in the cell material. When the vacuole membrane is broken, galectins pick up other sugar based molecules that are exposed by the damage. Unique galectins can pick up particular microbes such as S. Typhimurium. These can trigger pathways that will destroy the microbes.
 Another mechanism finds that the microbe’s vacuole is missing features that vacuoles usually have, even if there is no damage to them. There are special proteins that look at what the membranes are like in organelles such as ER, Golgi and cellular vesicles to see if they fit standards. These quality control proteins look at special amino acid combinations. The infected vacuoles often have less of these combinations, since special proteins are present from the microbe. This triggers a reaction.
Another mechanism finds that the microbe’s vacuole is missing features that vacuoles usually have, even if there is no damage to them. There are special proteins that look at what the membranes are like in organelles such as ER, Golgi and cellular vesicles to see if they fit standards. These quality control proteins look at special amino acid combinations. The infected vacuoles often have less of these combinations, since special proteins are present from the microbe. This triggers a reaction.
The specific compartments in the cell must have a particular structure or it is sensed as not normal. This includes ER, Golgi, lysosomes, lipid droplets and mitochondria, vesicles and vacuoles. All the normal ones have specific proteins that guard against destruction of the structure. T. gondii and Chlamydia trachomatis vacuoles don’t have them and so they are attacked.
Symbiotic Viruses in Vacuoles
 A further technique is exhibited with Leishmania spp. who live in macrophages and create a unique type of vacuole that appears to be very similar to a phagolysosome, a structure that usually is used by the cell to kill its contents. This microbe has a symbiotic virus that lives with it (Leishmania RNA virus LRV). It is the virus that tips the cell to their presence in the vacuole. The somehow senses the virus and produces multiple cytokines including interferon. It is possible that as the virus dies the microbe sends it out of the microbe.
A further technique is exhibited with Leishmania spp. who live in macrophages and create a unique type of vacuole that appears to be very similar to a phagolysosome, a structure that usually is used by the cell to kill its contents. This microbe has a symbiotic virus that lives with it (Leishmania RNA virus LRV). It is the virus that tips the cell to their presence in the vacuole. The somehow senses the virus and produces multiple cytokines including interferon. It is possible that as the virus dies the microbe sends it out of the microbe.
There are a very large number of microbes that live in vacuoles inside cells, and the cell has devised an equally large number of techniques to find them and destroy them. These mechanisms include complex cascades of receptors and stimulation of inflammatory cytokines. Many times, multiple different techniques are used at the same time.
Clever Microbes and Cells Fight Over Vacuoles
 In the constant battle between intelligent microbes and cells, the vacuole organelle has become a critical compartment. Perhaps, because of its complexity and its central role in many cellular functions, it has become a favorite for many microbes as a place to hide inside the cell. It is a surprising choice however, since the vacuole is a powerhouse of destruction for unwanted cellular material and microbes.
In the constant battle between intelligent microbes and cells, the vacuole organelle has become a critical compartment. Perhaps, because of its complexity and its central role in many cellular functions, it has become a favorite for many microbes as a place to hide inside the cell. It is a surprising choice however, since the vacuole is a powerhouse of destruction for unwanted cellular material and microbes.
The microbe is able to turn this powerhouse of destruction into a suitable living space where it can operate in the cell. Some symbiotic microbes live in vacuoles permanently. The cell struggles to develop ways to sense microbes living in the vacuole kill the microbes and retake the vacuole. This is another example of intelligent warfare between cells and microbes.
What is so surprising is that each microbe has developed different elaborate techniques by manufacturing complex molecules to interfere with complex cellular processes. The cell meanwhile has to be able to counter all of the many different microbes in different ways. Are these techniques prepared for all the different microbe species, just in case, before the microbe invasion? Or, does the cell invent them as they go along?
In either situation, both the microbes and cells show great ingenuity. They both have a strong will to survive and invent and counter many techniques used in this battle.