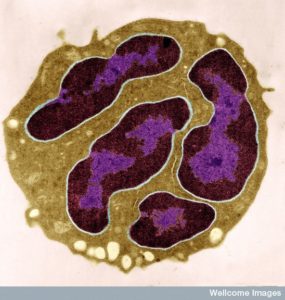
 Leukocytes, immune cells also known as white blood cells, use very complex modes of travel to navigate the vastly different environments of the various human organs. A variety of immune cells, including T cells and neutrophils, travel throughout the body, in and out of these tissues. There are thousands of different factors in the tissues that affect travel in difficult environments, as well as many local cells that aid travel. The goal of the complex migration of leukocytes is to position the immune cell to fight infection. The navigation can be very tricky and involves a vast array of different mechanisms. It is not clear how cells are able to know how to do it. Like many other processes described in these posts, the complex migration of leukocytes involves elaborate back and forth communication between many cells and the vast language of cytokines and chemical factors.
Leukocytes, immune cells also known as white blood cells, use very complex modes of travel to navigate the vastly different environments of the various human organs. A variety of immune cells, including T cells and neutrophils, travel throughout the body, in and out of these tissues. There are thousands of different factors in the tissues that affect travel in difficult environments, as well as many local cells that aid travel. The goal of the complex migration of leukocytes is to position the immune cell to fight infection. The navigation can be very tricky and involves a vast array of different mechanisms. It is not clear how cells are able to know how to do it. Like many other processes described in these posts, the complex migration of leukocytes involves elaborate back and forth communication between many cells and the vast language of cytokines and chemical factors.
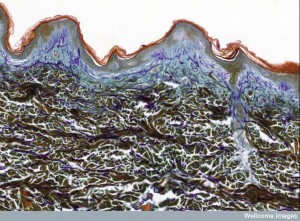 All cells exposed to the outside world, such as the skin, the lungs and GI tract, come in contact with many toxins, injuries and microbes. The immune system takes on all of these challenges, and its cells must position themselves accordingly.
All cells exposed to the outside world, such as the skin, the lungs and GI tract, come in contact with many toxins, injuries and microbes. The immune system takes on all of these challenges, and its cells must position themselves accordingly.
Usually, a small number of sentinel cells are present in any organ. But, when activated and alerted about danger, T cells, neutrophils and monocytes must travel to the site and into the organs. They need to be extremely active in their patrolling behavior in each different environment and, also, provide a very specific response upon arrival at the target. Their ability to migrate and rapidly enter the tissue is critical for the response.
At first the cells travel in the blood and then must grab onto the endothelial lining of the blood vessel using multiple adhesion molecules that allow rolling, tethering, and firm adherence. Then, they must travel between the endothelial cells and through the basement membrane into the extracellular space where they meet very different factors including chemical gradients that attract and repel them. They have to alter their shape as well as their direction many times. They can use their own actin-based propulsion to move, as well as travelling along non-cellular scaffolding structures. Once at the infected or damaged site they take stock and provide many repair functions.
Recently, new imaging devices have allowed the study of the navigation of live T cells and neutrophils, which has for the first time allowed fantastic research diretly observing these movements.
Migration
Individual cells have multiple different ways of movement for different three-dimensional situations. These are all provided by the operation of distinctive ways that actin-myosin motors, scaffolding structures, and moving tubules operate.
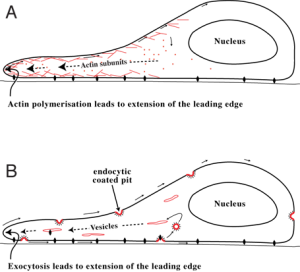
Amoeboid movement involves a round cell shape and a leading edge of the cell that moves. The pseudopod is a gliding form of movement that looks like an omoeba, maintaining the basic round shape, especially in the rear. Actin builds large molecules that rapidly move the leading edge forward into arms called pseudopods. Then other myosin motors contract in the middle of the cell. A large amount of different myosin motors in the back do not allow protrusion, which pushes movement forward.
Blebbing movement is another form that has contractions at the rear of the cell. This pushes cytoplasm forward forming round out-pouchings called blebs. This type of movement depends on outside adhesion.
Adhesions from the traveling cells attach to the lattice of the extracellular matrix allowing the cell to protrude forward. The attachment is by integrin molecules and a linking mechanism called the molecular clutch, which engages and disengages rapidly. This apparatus uses actin moving backwards and linking to integrin while the extra cellular matrix pushes the cell forward. Recent research shows that leukocytes walk stepwise with two feet below that alternatively form adhesions in different places. When the scaffolding proteins reach forward, these adhesions help push the cell.
Leukocytes (dendritic cells, neutrophils, and lymphocytes) use an actin protruding, integrin linking adhesion mode of movement some of the time and an independent form without attachment to extracellular structures other times. They can move along adhesive and non-adhesive surfaces at similar speeds.
When the tissue is very dense they 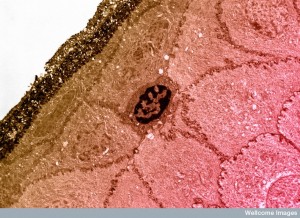 must make special actin-myosin contractions in the rear. Leukocytes are able to rapidly change their shape for different situations and travel quickly through many different environments.
must make special actin-myosin contractions in the rear. Leukocytes are able to rapidly change their shape for different situations and travel quickly through many different environments.
In low-density matrices, they can use less myosin activity. In highly dense material they form another structure called pearl-chain nuclei. Here they change the shape of the nucleus to get through small crevices rather than the typical large oval nucleus. Very recently, research has shown that leukocytes can move using scaffolding either when stimulated by external signals, or spontaneously with out external direction. In this latter case, the cell determines a specific direction to move.
Neutrophils Travelling
 Neutrophils clear debris and dangerous microbes from tissues. First they attach to the blood vessel near the inflammation on the other side and then they transit between the lining cells when immune cells outside the vessel signal to allow passage through the tight junctions. After this, they are confronted with the basement membrane. They pass through this membrane, again, when signals from local cells allow it.
Neutrophils clear debris and dangerous microbes from tissues. First they attach to the blood vessel near the inflammation on the other side and then they transit between the lining cells when immune cells outside the vessel signal to allow passage through the tight junctions. After this, they are confronted with the basement membrane. They pass through this membrane, again, when signals from local cells allow it.
The concept of many cells working together through complex communication was established with T cells in the the blood-brain barrier (see post on T cells in CSF ). It now appears that this complex signaling for leukocyte travel occurs in all body tissues. Back and forth signaling includes endothelial cells, pericytes, astrocytes, microglia, neurons and factors from the extracellular matrix. The neurons regulate the blood flow and signal to all of these other cells as well as the traveling immune cells.
In fact, each bodily organ has complex communication with multiple special cells that are part of the process of leukocytes entering tissues. These include intelligent activity between endothelial cells (see post Intelligent Gut Epithelial Cell), pericytes that surround the vessels, perivascular macrophages just inside the tissue, immune mast cells and the basement membrane itself. Signals and factors from all of these different cells determine not only the entrance into the tissue but also the transit through it.
Basement Membrane and Pericytes
 Small veins have basement membranes around them consisting of a very complex mesh of many long intertwined molecules. It holds together the wall of the blood vessel and generally stops cells like leukocytes from passing through. Inside the matrix are long cells called pericytes (many different sizes and densities in different regions) because they sit at the perimeter of the blood vessels.
Small veins have basement membranes around them consisting of a very complex mesh of many long intertwined molecules. It holds together the wall of the blood vessel and generally stops cells like leukocytes from passing through. Inside the matrix are long cells called pericytes (many different sizes and densities in different regions) because they sit at the perimeter of the blood vessels.
Another type of cell that intermittently surrounds the blood vessels, are immune macrophages which lie just beyond the basement membrane in skin, muscle and the brain. They have been recently discovered to send signals that regulate the tight junctions between the cells lining the vessel. These signals open them and allow space for neutrophils. These local macrophages sitting right on the outside of the vessel, also, secrete other chemicals that attract the leukocyte and give direction to the migration into the tissue.
This all occurs by complex signaling between the endothelial cells, the cells around the vessels and the leukocytes that are travelling through. Mast cells that also sit near the vessels—immune cells filled with histamine to stimulate inflammation—sense microbes and secrete histamine but also send other important cytokines that stimulate inflammation.
Priming Leukocytes for Battle
In addition to aiding their movement into the tissue, signals from these cells inside the tissue to the leukocyte give specific instructions about the problems to be faced in the local tissue, the specific microbes, problems in traveling in the tissue, and issues in clearing debris. In response to these messages, the leukocyte changes shape and becomes polarized. It also produces specific proteins and integrins. These signals are different in each tissue.
Traveling In the Tissue
 In the tissue, leukocytes are confronted with a wide variety of factors that hinder and aid their travels. These include factors in the extracellular matrix, factors from debris from damaged cells, a large number of cytokines (specific detailed signals with information) and chemokines (specific attracting or repelling functions).
In the tissue, leukocytes are confronted with a wide variety of factors that hinder and aid their travels. These include factors in the extracellular matrix, factors from debris from damaged cells, a large number of cytokines (specific detailed signals with information) and chemokines (specific attracting or repelling functions).
In the tissue, neutrophils communicate with each other, much as microbe communities do. They secrete signals that aid each other to find their appropriate place and complete their mission. Meanwhile, the tissue cells secrete many different attracting molecules that form a gradient toward the critical region and can guide the leukocytes a very long distance. Leukocytes have very complex sensory receptors that allow them to understand different gradients.Leukocytes, also, rely on specific scaffolds in each tissue to make traveling easier—in lymph nodes, liver, lung, skin, and the brain.
 Leukocytes know to leave the blood vessel in the direction of the infection. The new polarized shape helps the leukocyte to hone in the exact direction. They travel approximately 10um per minute and sometimes 30um per minute. When they arrive at the problem site they move more slowly. With communication among the leukocytes they gather in a cluster, until up to several hundred have arrived. In the case of injury, the cluster of leukocytes then walls off the area.
Leukocytes know to leave the blood vessel in the direction of the infection. The new polarized shape helps the leukocyte to hone in the exact direction. They travel approximately 10um per minute and sometimes 30um per minute. When they arrive at the problem site they move more slowly. With communication among the leukocytes they gather in a cluster, until up to several hundred have arrived. In the case of injury, the cluster of leukocytes then walls off the area.
First, scouts move in to form a small group. They produce strong attracting signals to other leukocytes. This signal makes the polar shape even stronger and pulls a large number of cells in. Leukocytes relay these messages to cells that are more distant to increase specific direction of movement and speed. Some of these cells might die in the process, which produces other signals. In certain infections a process of leukocyte swarming occurs where a large amount of cells produce large aggregates. Then the leukocytes remodel the extracellular matrix. Later other immune cells, like monocytes, appear at the periphery.
 Recent evidence confirms that migrating leukocytes use gradients, among several mechanisms, with higher densities closer to the site. One study showed that near a wound a hydrogen peroxide gradient was established extending a long ways into the tissue. Other studies have shown different tissues and injuries with a wide range of other chemical gradients. Some of these are produced in waves of different gradients. Leukocytes have to develop receptors for each of these.
Recent evidence confirms that migrating leukocytes use gradients, among several mechanisms, with higher densities closer to the site. One study showed that near a wound a hydrogen peroxide gradient was established extending a long ways into the tissue. Other studies have shown different tissues and injuries with a wide range of other chemical gradients. Some of these are produced in waves of different gradients. Leukocytes have to develop receptors for each of these.
Importantly, leukocytes are able to take in multiple competing signals and decide which are the most important. Each of these signals produces a different cascade of molecules within the cell to the nucleus. Some signals are telling the exact trouble spot and this will take precedence the closer they get to the site.
In some cases, there are repulsive signals, where the leukocytes take inflammation debris, turn around and travel in the opposite direction, back to the blood vessels and into lymph tissue and perhaps other organs. These leukocytes, then, stimulate a strong general signal from other parts of the body, which will sendl even more cells to travel to the trouble spot.
T Cells
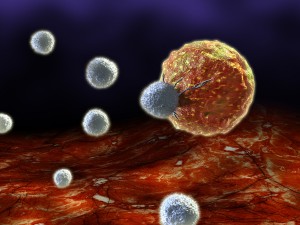 T cells, perhaps the brains of the immune system (see post Intelligent T cells), use all of the previously mentioned strategies, but also additional ones, since they must find the antigen in a large region where there are few of the cells they are seeking. They need even better homing and movement strategies since the target cells might, also, be in the midst of very dense inflammation sites. They must systematically scan whole regions and therefore, cannot use simple attractive schemes.
T cells, perhaps the brains of the immune system (see post Intelligent T cells), use all of the previously mentioned strategies, but also additional ones, since they must find the antigen in a large region where there are few of the cells they are seeking. They need even better homing and movement strategies since the target cells might, also, be in the midst of very dense inflammation sites. They must systematically scan whole regions and therefore, cannot use simple attractive schemes.
Like leukocytes, T cells, also, polarize their shape after entering tissue. But, unlike leukocytes they change their shape often and frequently stop before again moving in a new direction during which they become round again. These shapes can help moving through specific types of extra cellular matrix. 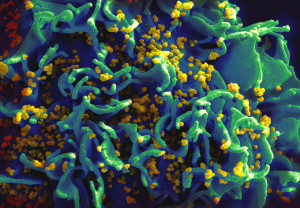
When they encounter an antigen, they stop moving completely and form a synapse with the target cell, either infected or cancerous. By connecting with the cell for minutes to hours they are able to destroy it. Cytotoxic killer T cells destroy a target cell about every 6 hours. After the killing they resume travelling rapidly scanning for more target cells.
Killer T cells have many different strategies in different tissues. In one example, when killer T cells are chasing herpes simplex virus in the skin, they divided into two types of cells with different modes of movement. A CD4 type actively migrated throughout the dermal tissue, while a CD8 type changed its shape to that of a dendritic cell with many long arms and crawled in the epidermis very slowly between the cells. The CD4 type went into the blood, while the CD8 stayed in the epidermis, where it painstakingly found skin cells that had the virus antigen on its surface.
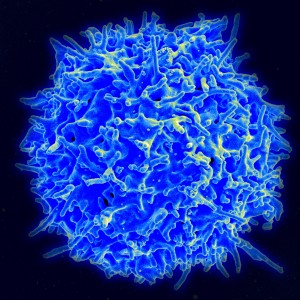 Several important signaling pathways for stimulation and inhibition affect T cells mobility, and these can impair its mobility as well as help it. In addition to other activation patterns of T cells, these signals greatly affect its activity. One signal increases the movement to the extent that it doesn’t form proper synapses with other immune dendritic cells, and therefore, doesn’t get the antigen information.
Several important signaling pathways for stimulation and inhibition affect T cells mobility, and these can impair its mobility as well as help it. In addition to other activation patterns of T cells, these signals greatly affect its activity. One signal increases the movement to the extent that it doesn’t form proper synapses with other immune dendritic cells, and therefore, doesn’t get the antigen information.
Another signal reduces the T cells speed of operation in the pancreas making the necessary contacts with other cells much less efficient. Another example is in the spleen where T cells can become exhausted causing very serious inflammatory disease.
Many Types of T Cell Travel
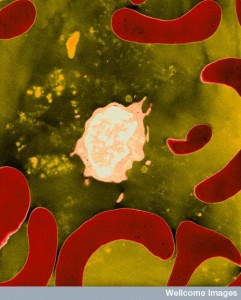 In tissues, T cells generally don’t use gradients for movement, since they are surveilling large tissues for a small amount of microbe antigen. Instead they commonly use a modification of the amoeboid random walk.
In tissues, T cells generally don’t use gradients for movement, since they are surveilling large tissues for a small amount of microbe antigen. Instead they commonly use a modification of the amoeboid random walk.
In the skin, the brain and in some tumors, they crawl along extra cellular matrix (ECM) structures, where there is cytokine and receptor communication between the T cell and the extra cellular matrix. Various fibers in the extra cellular matrix communicate with diverse T cells in each tissue. In some, back and forth communication changed both the extra cellular matrix itself and the shape of the T cells. The ECM can either help or hurt the travels of the T cells in different types of tissue.
Another type of random walk of the T cell observed in the brain is called Levy walks. In this type of travel there is amoeboid walk with intermittent high velocity runs that allow the T cell to rapidly get much further into the tissue. This type of walk is much better at finding hidden infected cells deep in the tissue. Special chemokine signals increase the speed of this type of movement increasing the ability to find the target cells. 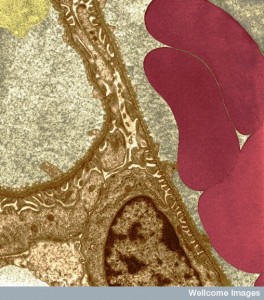
Another mechanism of travel for the T cell is contacting other immune macrophages and dendritic cells that it meets along the way in the tissue. Contact with these other cells can be slow or rapid, where the T cell apparently gets information to continue its search by accumulating different signals.
Thus, T cells can use scaffolds of tissue and devise specific types of movement to help find the targets deep in specific tissues.
A complex technique involves the T cell accumulating and summing many different signals from a variety of cells along the way. An example of this occurs in autoimmune encephalomyelitis where multiple brief contacts with other cells help gather information for the T cells eventually effective search. To store this information, the T cell transfers a specific factor from the cytoplasm into its nucleus. The nucleus accumulates various signals from other cells and helps navigation. Gathering information by many brief contacts with cells along the way was also observed when chasing a cancer.
Cancer Cells Migration
 Several other mechanisms of movement were recently described in cancer cells. To get through very tight spaces cancer cells stimulate a water flow that is like a sailboat mechanism. At the leading edge, the membrane takes in water and ions through channels called aquaporins. This flow of water is then pumped out the back of the cell, pushing the cell forward.
Several other mechanisms of movement were recently described in cancer cells. To get through very tight spaces cancer cells stimulate a water flow that is like a sailboat mechanism. At the leading edge, the membrane takes in water and ions through channels called aquaporins. This flow of water is then pumped out the back of the cell, pushing the cell forward.
Another mode of travel in cancer cells involves “chasing” healthy cells. The ordinary cell alters its mode of travel to try to escape from the cancer cell, which increases the speed of both.
Recently, a third travel mode was crawling along blood vessels.
Complex Migration of Leukocytes
As more is learned about cells of all types, what is striking is the extremely complex communication that pervades all physiology. During movement throughout the body, very complex communication occurs between a wide variety of cells, neurons, endothelial cells, basement membrane, pericytes that sit around blood vessels, and each type of immune cell. Each tissue has its own type of communication about the unique issues in traveling through and the language is universally complex.
 With many different types of movement available, leukocytes, including T cells, are able to use a wide variety of different techniques to enter into the specific organs of the human body and travel to the critical site. Techniques include changing shapes and using the leading edges of the cell in different ways. Leukocytes can decide to suddenly swarm near a target. They use chemical gradients, cytokine signaling with cells and extracellular matrix. Cells are able to alter their shape and modes of transport as needed.
With many different types of movement available, leukocytes, including T cells, are able to use a wide variety of different techniques to enter into the specific organs of the human body and travel to the critical site. Techniques include changing shapes and using the leading edges of the cell in different ways. Leukocytes can decide to suddenly swarm near a target. They use chemical gradients, cytokine signaling with cells and extracellular matrix. Cells are able to alter their shape and modes of transport as needed.
The leukocyte uses the back and forth communication with many other cells to analyze the best way to traverse the specific terrain as well as the specific response to the target problem.
With so much sophistication in communication and in the adaptations to different tissues, how can anyone say that this is not an intelligent process? How can anyone think that leukocytes are not intelligent?