 The brain has many layers of protection from microbes. But, with great ingenuity microbes have mechanisms of traversing the tortuous multi layered pathways into the brain. This trip takes many different stages, and each stage needs highly specific complex machinery and signaling with complex molecules. Microbes are able to send their own complex signals and trigger immune cytokines and neurotransmitter signaling for the own advantage at every step of the complex journey. The microbe tricks for entering the brain include specific surface molecules that can attach to vessel lining cells; ways of fooling immune cells at their own game; and intelligent manufacture of new signals that have effects on both brain and immune cells.
The brain has many layers of protection from microbes. But, with great ingenuity microbes have mechanisms of traversing the tortuous multi layered pathways into the brain. This trip takes many different stages, and each stage needs highly specific complex machinery and signaling with complex molecules. Microbes are able to send their own complex signals and trigger immune cytokines and neurotransmitter signaling for the own advantage at every step of the complex journey. The microbe tricks for entering the brain include specific surface molecules that can attach to vessel lining cells; ways of fooling immune cells at their own game; and intelligent manufacture of new signals that have effects on both brain and immune cells.
The Layers of Protection
 There are many specific barriers to avoid traffic into the brain. The blood brain barrier is a term used for multiple different obstructions between particles and microbes in the blood and the extracellular fluid that lies between brain cells as well as the cerebrospinal fluid that surrounds the brain. It consists of special tight junctions between cells lining the blood capillaries, a thick basement membrane and a thick and continuous layer of astrocyte end feet that fully surrounds the blood vessels.
There are many specific barriers to avoid traffic into the brain. The blood brain barrier is a term used for multiple different obstructions between particles and microbes in the blood and the extracellular fluid that lies between brain cells as well as the cerebrospinal fluid that surrounds the brain. It consists of special tight junctions between cells lining the blood capillaries, a thick basement membrane and a thick and continuous layer of astrocyte end feet that fully surrounds the blood vessels.
The capillaries in the brain, the smallest blood vessels, are different from 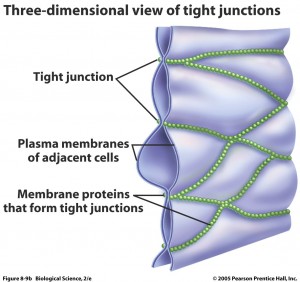 usual capillaries in that the lining cells are very tightly joined together not allowing diffusion of microbes, large particles and hydrophilic (water soluble) particles. They do allow small hydrophobic molecules in, such as oxygen, carbon dioxide and hormones. Lining cells do actively transport important metabolites like glucose.
usual capillaries in that the lining cells are very tightly joined together not allowing diffusion of microbes, large particles and hydrophilic (water soluble) particles. They do allow small hydrophobic molecules in, such as oxygen, carbon dioxide and hormones. Lining cells do actively transport important metabolites like glucose.
Just outside of the vessels is the basement membrane of the extracellular space—a solid sheet of molecules made of collagen, laminin and many other complex molecules. Then, there is a layer of astrocyte end feet,  which covers all surfaces of the blood vessels and determines the blood flow (the measure of MRI).
which covers all surfaces of the blood vessels and determines the blood flow (the measure of MRI).
Only special signals and damage can cause leaks in this complex three-layered structure.
There are more complex structures. The Perineural Nets are a mesh of large molecules that cover all neurons. These consist of proteoglycans, tenascin and linking proteins and are critical for synapses and neuroplasticity. Then, there is the dense interstitial matrix not attached to the basement membrane or perineural nets, but floating freely.
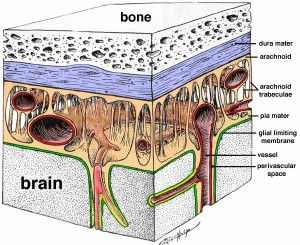 The cerebral spinal fluid flows in the middle of three membrane barriers called the meninges.
The cerebral spinal fluid flows in the middle of three membrane barriers called the meninges.
To get into the brain and infect a neuron, all of these layers have to be breached. Each needs its own intelligent tricks. The microbe has to have dozens of different effective tricks that can be used in sequence.
Despite Barriers Microbes Enter the Brain
Clever microbes, however, do enter and exit the brain and neurons. Microbes can hijack and hide in immune cells. Microbes can even change behavior of animals to their advantage.
Many different kinds of bacteria and virus attack the brain. Worldwide, a large number of people die from bacterial meningitis and malaria. Trypanosomiasis, neurocysticercosis, rabies, measles, polio, HIV, and henipavirus (from bats) all enter the brain.
The blood brain barrier stops many of the ordinary immune cells from entering the brain. A previous post shows how intelligent T cells, as well as other immune cells, provide surveillance in the cerebrospinal fluid and control the inflammation response in the brain. When neurons are infected, they don’t display the MHC signals on the cell surface and therefore T cell don’t react to them (see post for discussion of the way T cells read MHC signals on cells). There are, also, a large amount of cytokine signals in the brain that stop inflammation.
Microbes are able to build a niche in specific regions of the brain, based on special characteristics of their entry process, and the special receptors for specific microbes that exist on the surface of neurons and glia. A previous post noted that in different brain regions different cells have individually developed their own local immune systems targeted at very specific microbes.
When microbes are able to enter the brain, some attack neurons; others such as polio, attack oligodendrocytes, the cells that make myelin. Leprosy attacks Schwann cells that surround peripheral neurons.
Entry Through Lining Cells Into Blood
 One of the main ways that products are taken into the blood is through an active process where cells’ membranes merge with a sac (vesicle) transporting it through the cell and secreting it on the other side into the blood stream (endocytosis and exocytosis). The blood vessel lining cells, which protect the brain, do not take up vesicles as lining cells do in other parts of the body. They are very tightly connected together. In one way around the problem of crossing the lining cells, malaria doesn’t cross the blood brain barrier, but attaches to blood cells and vessels and causes trouble from there through signaling.
One of the main ways that products are taken into the blood is through an active process where cells’ membranes merge with a sac (vesicle) transporting it through the cell and secreting it on the other side into the blood stream (endocytosis and exocytosis). The blood vessel lining cells, which protect the brain, do not take up vesicles as lining cells do in other parts of the body. They are very tightly connected together. In one way around the problem of crossing the lining cells, malaria doesn’t cross the blood brain barrier, but attaches to blood cells and vessels and causes trouble from there through signaling.
Some microbes disturb the blood brain barrier (BBB) such as adenovirus or Nipah virus, which cause hemorrhage with brain infection. Some microbes make a specific molecule on their surface that attaches to the lining cells and then a special plasmid that causes disruption through signaling.
Anthrax makes a toxin that breaks open the tight junctions between the lining cells. It attaches to the lining cells and stimulates the immune factors in the cells, NF-kappa-beta and interferon regulatory factor that cause inflammation.
The monkey HIV, called SIV (for simian) enters the lining cells and multiplies inside the cell. This process does not use T cells as HIV does. The mechanism involves proteoglycans, discussed in the post on Extra Cellular Matrix. Although most endothelial lining cells don’t bring in vesicles, a small class of cells in the basement membrane, called pericytes, do. These bring in HIV virus and transport it without multiplication all the way to the other side and into the brain.
Entry With Infected White Blood Cells
 Normally, white blood cells (WBC) cross the BBB to observe for invasions by microbes. Although the brain was considered to have no immune cells previously, the post on Intelligent T cells shows that there are many blood cells in the CSF and some do cross into the brain. It is hard to observe a small number of cells in the brain. When inflammation is started, there are many more WBCs that cross. They cross by attaching to the venule (smallest veins) then, in steps, and with different complex mechanisms, traverse lining cells and, then, basement membrane cells. Different regions have different laminin structures and are part of this process.
Normally, white blood cells (WBC) cross the BBB to observe for invasions by microbes. Although the brain was considered to have no immune cells previously, the post on Intelligent T cells shows that there are many blood cells in the CSF and some do cross into the brain. It is hard to observe a small number of cells in the brain. When inflammation is started, there are many more WBCs that cross. They cross by attaching to the venule (smallest veins) then, in steps, and with different complex mechanisms, traverse lining cells and, then, basement membrane cells. Different regions have different laminin structures and are part of this process.
Cytokine communication occurs between immune and brain cells and these also help allow WBCs to cross. When the basement membrane opens from a special signal, WBCs can pass by the astrocytes end feet layer, a significant barrier surrounding blood vessels. Crossing these multiple barriers involve multiple different laminins and many different signaling cytokines.
Herpes, HIV, SIV, and measles reproduce in T cells and in monocytes and are carried into the brain by these immune cells. A cytokine signal from astrocytes is triggered by these viruses, which calls for more monocytes. These monocytes infect macrophages and microglia. Those that are near neurons cause inflammation that disturb the synapses.
The bacteria listeria monocytogenes and the infamous Toxoplasma gondii, which causes mental effects in animals (including humans), also use monocytes to cross. These monocytes are taken up in the intestine. T. gondii, then, enters the neurons directly and they can stay there for many years (entire life).
There are many different other cytokine signals and techniques involving white blood cells that are used by many other microbes including Trypanosoma, causing sleeping sickness. Through signaling, the intelligent T cells help a variety of microbes to enter.
West Nile virus is stopped by Toll-like receptors, which trigger immune factors like TNF (tumor necrosis factor.) TNF stops blood cells from entering. Even with very few in the blood, some of the virus enter and infect neurons especially in the basal ganglia, thalamus, brainstem and cerebellum.
Entry From Blood Vessels
 Even without entering the brain proper, malaria parasites in red blood cells create signals that make the RBC stick to the vessel lining cells. This affects blood flow. A new finding is that during the infection with malaria, many very small vesicles called micro-particles are released from the RBCs, WBCs, platelets and vessel lining cells. These particles bind to macrophages and stimulate many signaling cytokines causing inflammation and malaria infection in the brain.
Even without entering the brain proper, malaria parasites in red blood cells create signals that make the RBC stick to the vessel lining cells. This affects blood flow. A new finding is that during the infection with malaria, many very small vesicles called micro-particles are released from the RBCs, WBCs, platelets and vessel lining cells. These particles bind to macrophages and stimulate many signaling cytokines causing inflammation and malaria infection in the brain.
Entry Through Blood Brain Barrier And Subarachnoid CSF
In the subarachnoid space and ventricles, microbes can travel from blood to the CSF. Most use the meningeal blood vessels.
The lining cells are tightly joined, but they allow some large molecules through. The bacteria strep, pneumonia, Neisseria meningitides, haemophilus influenza and the fungus cryptococcus cause inflammation in the CSF. These are possibly taken across the lining cells in vesicles. The trick all of these pathogens use is to have a binding factor that is very similar to the surface of platelets and it stimulates specific cytokine signals (platelet activating factor, PAF). PAF also triggers neuronal cell death.
There are many other mechanisms used by other microbes including stimulating a neurotransmitter receptor that causes the scaffolding to change, allowing gaps in the lining cells.
In Ventricles
 Some bacteria can cross the choroid plexus lining cells into the brain’s ventricles. This is done by triggering specific signals that cause the cells to take up and transmit vesicles. At times these reactions are quite complex with very specific cytokine signaling between microglia, macrophages, and T cells. These immune cells are prodded by signaling to disrupt the lining with inflammatory responses. Monocytes with HIV inside can also cross the choroid plexus.
Some bacteria can cross the choroid plexus lining cells into the brain’s ventricles. This is done by triggering specific signals that cause the cells to take up and transmit vesicles. At times these reactions are quite complex with very specific cytokine signaling between microglia, macrophages, and T cells. These immune cells are prodded by signaling to disrupt the lining with inflammatory responses. Monocytes with HIV inside can also cross the choroid plexus.
Other microbes stay in the choroid plexus cells and from there send signals that cause inflammation in the ventricles. Trympanosome brain invasion occurs in this way.
In Axons
Some microbes travel in the axons.
 Peripheral nerves in the body are protected because lining cells called the perineurium and endoneural vessels surround them. The axon is protected except at the end and there molecules can be taken into the cell and transported along the axon back to the nucleus. These molecules can be a lead in point for microbes to enter the neuron.
Peripheral nerves in the body are protected because lining cells called the perineurium and endoneural vessels surround them. The axon is protected except at the end and there molecules can be taken into the cell and transported along the axon back to the nucleus. These molecules can be a lead in point for microbes to enter the neuron.
Viruses with an envelope—rabies and herpes—are similar to a vesicle, which merges with the membrane and releases the virus into the cell. Those without an envelope—polio and adenovirus—break open the cell membrane. There are many different ways that the vesicles and envelopes are introduced into the cell using scaffolding machinery.
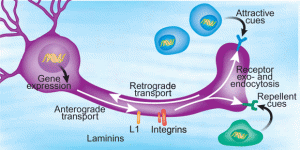 Those viruses that don’t use their own envelope, but rather a cellular vesicle, leave no immune traces. The trace can come from the envelope, which can be detected by immune receptors. The herpes virus is hard to trace and can stay for long periods inside neurons. Some of the vesicles, called endosomes, are transported by microtubule motors.
Those viruses that don’t use their own envelope, but rather a cellular vesicle, leave no immune traces. The trace can come from the envelope, which can be detected by immune receptors. The herpes virus is hard to trace and can stay for long periods inside neurons. Some of the vesicles, called endosomes, are transported by microtubule motors.
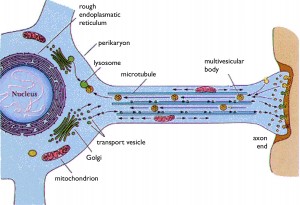 Rabies is carried by an endosome (vesicle) retrograde from the tip of the axon all the way back to the nucleus. The virus lives first in skeletal muscle cells, then meets the axon at the neuromuscular junction and then can travel all the way up the spinal cord into the CNS. It can also start in sensory or autonomic nerves, as well. In the autonomic system it travels to the salivary gland where it joins saliva and infects another human. These endosomes are kept at specific pH to stop the release of the virus until it has travelled all the way to the nucleus.
Rabies is carried by an endosome (vesicle) retrograde from the tip of the axon all the way back to the nucleus. The virus lives first in skeletal muscle cells, then meets the axon at the neuromuscular junction and then can travel all the way up the spinal cord into the CNS. It can also start in sensory or autonomic nerves, as well. In the autonomic system it travels to the salivary gland where it joins saliva and infects another human. These endosomes are kept at specific pH to stop the release of the virus until it has travelled all the way to the nucleus.
Some travel fast and some slow. Polio is transported by the dynein motors on microtubules rapidly, in addition to having a different separate very slow transport.
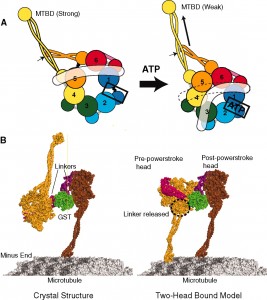 Recently, it was shown that when the herpes virus takes over the dynein motor it actually alters the energy mechanism (ATP source) and speeds it up. It is not a passive passenger on the dynein motor but grabs the wheel and increases the throttle. It moves very rapidly from the skin to the nucleus and then back later.
Recently, it was shown that when the herpes virus takes over the dynein motor it actually alters the energy mechanism (ATP source) and speeds it up. It is not a passive passenger on the dynein motor but grabs the wheel and increases the throttle. It moves very rapidly from the skin to the nucleus and then back later.
Herpes can infect peripheral ganglia. There are many different mechanisms that allow herpes to travel along its complex route. It has several different ways of travelling cell to cell. Another complex mechanism allows it to enter into the axon. After multiplying in the sensory neuron, it then travels again by retrograde transport into the CNS. It can travel in pieces or as a whole in vesicles, which are transported along microtubules. A totally different mechanism provides herpes with anterograde transport to return.
In addition to viruses, bacteria can also infect neurons, such as L. monocytogenes. It travels in the trigeminal nerve and causes brain stem infection.
Entry Through Olfactory System
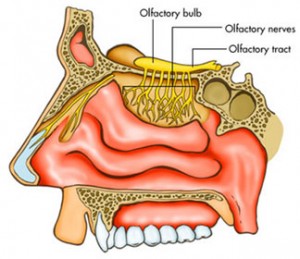 Olfactory neurons’ dendrites directly contact the outside of the CNS. These neurons have dendrites in the external environment and axons in the brain. Many viruses travel this route, such as herpes, Borna, and paramyxovirus. They travel by axon to the olfactory bulb. By retrograde transport they can enter the limbic system and the monoamine systems of the brainstem. One example goes to the serotonin center, the Raphe nucleus and causes life long low serotonin.
Olfactory neurons’ dendrites directly contact the outside of the CNS. These neurons have dendrites in the external environment and axons in the brain. Many viruses travel this route, such as herpes, Borna, and paramyxovirus. They travel by axon to the olfactory bulb. By retrograde transport they can enter the limbic system and the monoamine systems of the brainstem. One example goes to the serotonin center, the Raphe nucleus and causes life long low serotonin.
Some bacteria from the nose can go to the subarachoid space and cause meningitis. Amoeba can also enter this way. Naegleria fowleri has been in the news recently for entering the brain of several children who swam in infested water (one recently as north as Minnesota).
Microbes Living in Neurons
 Ganglia are not as protected as CNS and ganglia cells come in contact with blood and interstitial matrix and therefore immune T cells that stop microbes. Therefore, some viruses are stuck inside of peripheral nerves and cannot get to the brain.
Ganglia are not as protected as CNS and ganglia cells come in contact with blood and interstitial matrix and therefore immune T cells that stop microbes. Therefore, some viruses are stuck inside of peripheral nerves and cannot get to the brain.
Ssome viruses that make it into the brain stay there in a hibernating state, but then re activate and travel to the periphery to go to the skin in order to spread to others.
Several viruses in the CNS are secreted in vesicles from neurons and then taken up by microglia, where they are broken up into pieces of proteins that can be presented to T cells in MHC. The T cell produces signals, such as interferon gamma, that stops the reproduction of the virus. It can also induce nitrous oxide from microglia that is an antibiotic.
Other microbes in neurons, such as rabies, trigger signals that suppress immune activity such as HLA-G1 that protects them.
By complex mechanisms, some viruses kill the neuron, or alter its function. In other cases, when T cell’s signals attempt to control the infection, they can, instead, alter and impair a neuron’s function. This occurs in malaria and HIV.
Microbes in Different Brain Regions Alter Behavior
Behavior in animals, including humans, can change based on where the microbe attacks the brain. There are many different behaviors caused by microbes.
Examples include aggressive behavior from rabies in the brainstem, thalamus and hippocampus; and T. gondi in prefrontal cortex causing mice to expose themselves to cats and suicidality in humans.
Tick born encephalitis infests motor neurons causing paralysis and epilepsy. Many other microbes cause considerable amount of epilepsy in large parts of the world. Von Economo encephalitis cause lethargy, whereas trypanosomiasis cause insomnia. Studies of these microbes first elucidated the hypothalamic sleep wake centers.
Zoster infests neurons that induce pain in dorsal root ganglia neurons. Reproduction of these viruses is controlled by interferon gamma.
Microbe Tricks for Entering the Brain
 Microbes traverse a long tortuous route from the outside through skin, mucous, blood vessels, the many barriers to the brain, and the vast complexity of the neuron. Invasion of the brain involves many different complex mechanisms each of which has to be in sequence.
Microbes traverse a long tortuous route from the outside through skin, mucous, blood vessels, the many barriers to the brain, and the vast complexity of the neuron. Invasion of the brain involves many different complex mechanisms each of which has to be in sequence.
What is quite remarkable is that in different parts of the journey, microbes use very specific complex cytokine signals and manufacture very specific shaped molecules. It is remarkable that a tiny cell, or a virus ( which is, virtually, just a piece of DNA or RNA) can make these multiple different signaling molecules, or the specific shapes needed, or utilize complex mechanisms that overtake the energy supply of the dynein motors while travelling on microtubules. Specific microbes have even devised specific ways of behaving in different brain regions.
How can we say that these microbes do not exhibit intelligent behavior?