 T cells are the masters of the immune system. Through active communication with the brain they maintain balance between the immune and nervous systems. T cells respond to situations by regulating all other immune cells through signaling, either with secreted molecules or by direct contact. T cells have many very specific talents. They are one of only two cells that can edit their own DNA to make many unique receptors to pick up evolving microbes. The other cell that edits its own DNA to is B-lymphocytes when making unique antibodies. T cells direct B-lymphocytes to produce even better antibodies through direct communication between the two cells (a process called “hypermutation”).
T cells are the masters of the immune system. Through active communication with the brain they maintain balance between the immune and nervous systems. T cells respond to situations by regulating all other immune cells through signaling, either with secreted molecules or by direct contact. T cells have many very specific talents. They are one of only two cells that can edit their own DNA to make many unique receptors to pick up evolving microbes. The other cell that edits its own DNA to is B-lymphocytes when making unique antibodies. T cells direct B-lymphocytes to produce even better antibodies through direct communication between the two cells (a process called “hypermutation”).
T cells can suddenly alter themselves to become different kinds of cells. One dramatic change occurs when they are activated to fight particular microbes. The activation occurs through a decision-making process involving communication with dendritic cells. Immune dendritic cells present material they have picked up for evaluation by T cells. When the material is from a dangerous microbe, cancers or damaged cells, T cells activate.
 T cells can produce an army of powerful killer cells and many other T helper cells. To become a killer cell, the entire metabolism is altered by using ordinary metabolic cycles to signal genetic networks. Killer T cells are able to reproduce very rapidly to create an army of fighting cells. They become faster, larger, and able to travel even without oxygen and nutrients in order to attack other cells. They produce the immune synapse (see post), which is a powerful connection that rapidly kills the other cells.
T cells can produce an army of powerful killer cells and many other T helper cells. To become a killer cell, the entire metabolism is altered by using ordinary metabolic cycles to signal genetic networks. Killer T cells are able to reproduce very rapidly to create an army of fighting cells. They become faster, larger, and able to travel even without oxygen and nutrients in order to attack other cells. They produce the immune synapse (see post), which is a powerful connection that rapidly kills the other cells.
The other change is when T cells differentiate into many different types of T helper cells. T helper cells have many functions. A post described how signals from food trigger the production of very special T cells that inhibit reactions to food that would otherwise produce massive daily food allergies. Another helper cell is produced during inflammation so that killer cells will be directed to stop their aggressive fighting when the battle is won. Otherwise, killer cells would then turn against the body itself. T helper cells direct other immune cells in each type of inflammation. T helper cells in the cerebrospinal fluid inhibit other immune cells from producing inflammation in the brain.
It is remarkable that T cells can produce so many different kinds of cells. This post describes recent work about very specific receptors called C lectin receptors or CLRs. These receptors are very active in the signaling pathways that determine how the T cell will make very particular helper cells. A previous post described great complexity in pattern recognition receptors PRRs in general. These receptors are very highly regulated and produce many important effects. These new CLR pattern receptors are even more complex and interact with many other receptors to produce highly specific and very variable effects for many different kinds of microbe attacks.
Many Different Helper Cells
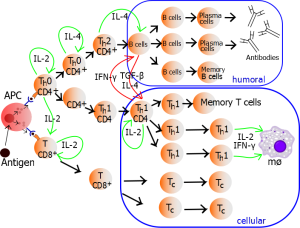 Different types of T cells are triggered for a wide range of jobs. Previous posts showed how particular T cells are triggered to fight food allergies. Other T cells are needed to damp down killer cells after they have vanquished microbes but before they become destructive to human cells. In fact, there are many different varieties of killer cells and T helper cells based on what microbe has invaded. Recently, signals have been found that produce a wide variety of different killer T cells and helper T cells.
Different types of T cells are triggered for a wide range of jobs. Previous posts showed how particular T cells are triggered to fight food allergies. Other T cells are needed to damp down killer cells after they have vanquished microbes but before they become destructive to human cells. In fact, there are many different varieties of killer cells and T helper cells based on what microbe has invaded. Recently, signals have been found that produce a wide variety of different killer T cells and helper T cells.
T cells activation to fight dangerous microbes, cancers and toxins is a result of group conversations. Immune dendritic cells and macrophages present material they have picked up to T cells to determine whether they represent a danger. For T cells to decide to activate, they need back and forth communication with these various dendritic cells. It now has been shown that specific receptors on dendritic cells do more than trigger activation of T cells to become armies of killer cells. In fact, specific lectin receptors (C type lectin receptors or CLRs) also trigger the specific type of T helper cells that are needed for each situation. In fact these CLRs act in conjunction with other receptors as well. These produce particular cytokine signals that influence differentiation of T cells.
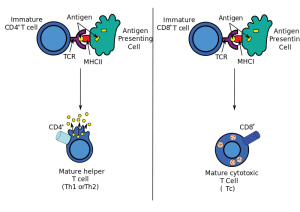 To fight dangerous microbes, CD4+ T cells change into many kinds of T helper cells based on the type of inflammation that is occuring. These T helper cells coordinate other immune cells to create a particular defense. Dendritic cells help orchestrate the production of these particular helper T cells. Dendritic cells find particular patterns of the invading microbe and then produce signals that alter T cell gene networks producing the particular T helper cell. This cell then produces a wide variety of cytokines to control other types of cells.
To fight dangerous microbes, CD4+ T cells change into many kinds of T helper cells based on the type of inflammation that is occuring. These T helper cells coordinate other immune cells to create a particular defense. Dendritic cells help orchestrate the production of these particular helper T cells. Dendritic cells find particular patterns of the invading microbe and then produce signals that alter T cell gene networks producing the particular T helper cell. This cell then produces a wide variety of cytokines to control other types of cells.
Not all of these T helper cells are known, but there is increasing evidence of a wide variety. For example T1 helper cells deal with intracellular microbes, T 2 deal with extracellular microbes and T17 are special for fungus. Each of these produces different cytokine sets that create a particular milieu. Another follicular type Tph is related to long term immunity. T 9 and T 22 interact with other specific microbes.
A Large Number of Receptors
 This post will discuss the newly discovered receptors or CLRs and how they serve as signals that are triggered on dendritic cells. They are defined by a particular section of the protein receptor called C type lectin—a molecule that recognizes many types of other molecules. These regions of the receptors recognize many different carbohydrates that are on the surface of various microbes, including fucose, mannose, sialic acid and many others. CLRs are mostly on dendritic cells and macrophages, but some are also on skin cells keratinocytes, B-lymphocytes and even white blood cells such as neutrophils.
This post will discuss the newly discovered receptors or CLRs and how they serve as signals that are triggered on dendritic cells. They are defined by a particular section of the protein receptor called C type lectin—a molecule that recognizes many types of other molecules. These regions of the receptors recognize many different carbohydrates that are on the surface of various microbes, including fucose, mannose, sialic acid and many others. CLRs are mostly on dendritic cells and macrophages, but some are also on skin cells keratinocytes, B-lymphocytes and even white blood cells such as neutrophils.
CLRs are different from other pattern recognition receptors that were described in a previous post. These are part of a process that particularly targets and takes apart dangerous microbes and then alters important signals in the immune pathways. CLR receptors sense particular dangerous microbes, as well as cancers. These are based on particular patterns of sugars sometimes called “carbohydrate fingerprints”. Bacteria and other microbes have very particular patterns of molecules that are distinct from humans. Viruses also create unique patterns even though they use human sugars for their envelope, such as mannose. Cancers also have unique patterns.
Dectin 1 Receptor Responds to Fungus
 The particular CLR dectin 1 identifies fungus that are both dangerous and friendly resident fungi in the gut. They identify β-glucans that are picked up by dendritic cells and special gut immune Langerhans cells. Once triggered, they help produce T17 helper cells on the skin and T1 below the skin. Dectins form synapses with the β-glucan molecules. This triggers multiple kinase enzymes in pathways and the cytokines NF-κB pathway in several different ways. Many other cytokines are brought into this complex process including IL-6 and IL-23. IL-1β and IL-12p40 are also part of this process triggered by dectin as well as other signals. These have to overcome inhibition by IL1B and IL12B. All of these together produce differentiation of T cells into T17 helper cells.
The particular CLR dectin 1 identifies fungus that are both dangerous and friendly resident fungi in the gut. They identify β-glucans that are picked up by dendritic cells and special gut immune Langerhans cells. Once triggered, they help produce T17 helper cells on the skin and T1 below the skin. Dectins form synapses with the β-glucan molecules. This triggers multiple kinase enzymes in pathways and the cytokines NF-κB pathway in several different ways. Many other cytokines are brought into this complex process including IL-6 and IL-23. IL-1β and IL-12p40 are also part of this process triggered by dectin as well as other signals. These have to overcome inhibition by IL1B and IL12B. All of these together produce differentiation of T cells into T17 helper cells.
When manufactured T17s have errors, excessive inflammation can occur and this must be controlled. These are regulated in a very complex way using the above-mentioned cytokines as part of complex pathways.
 T17 has to be regulated against having too much or too little. Another mechanism affects T17by increasing use of interferon-β (IFNβ)38. This decreases IL-1β, by increasing IL-27 and both together affect T17.
T17 has to be regulated against having too much or too little. Another mechanism affects T17by increasing use of interferon-β (IFNβ)38. This decreases IL-1β, by increasing IL-27 and both together affect T17.
Another mechanism used by fungi and dangerous bacteria trigger inflammasomes. A previous post described these large multi protein machines that regulate specific types of inflammation. This particular inflammasome is triggered by caspase 1 and IL-1β. Caspase is triggered by dectin. Another inflammasome is triggered by dectin and caspase 8.
A different mechanism uses remodeling of chromatin structures. This genetic network makes IL12A, and in particular, a special subunit of the molecule called IL-12p35. The cytokine NF-κB39 activates these special genetic processes. In this process triggered also by dectin, T1 cells are altered to T2, which work against the fungus.
CD209 Most Studied CLR
 This receptor has many names since it has been found in many varied processes. It is also called by the strange name DC-SIGN (DC-specific ICAM3-grabbing non-integrin). Triggering this receptor by carbohydrate molecules on microbes, stimulates many pathways that interact with a wide variety of other pattern recognition receptors. Mycobacteria tuberculosis, viruses, parasites, helicobacter pylori, and fungi all trigger this receptor. With both types of receptors operating T1, T17, T2 and Tph are all involved in responses.
This receptor has many names since it has been found in many varied processes. It is also called by the strange name DC-SIGN (DC-specific ICAM3-grabbing non-integrin). Triggering this receptor by carbohydrate molecules on microbes, stimulates many pathways that interact with a wide variety of other pattern recognition receptors. Mycobacteria tuberculosis, viruses, parasites, helicobacter pylori, and fungi all trigger this receptor. With both types of receptors operating T1, T17, T2 and Tph are all involved in responses.
The sugar mannose on microbes helps regulate T1 and T17 responses. HIV, Ebola and Hep C as well as tuberculosis have multiple mannose structures that trigger these receptors. Fungus cell walls contain mannan, a form of mannose. All of these trigger dectin, and other pattern receptors TLR2 and TLR4 as well. These pathways send out elaborate cytokine programs including IL-12p35, IL-12p40 and IL-6. These pathways produce a complex multi protein complex in some ways similar to an inflammasome called a RAF1 signalosome. This creates complex pathways that eventually induce T1 and T17 T cells. Multiple different cytokines get into the act such as IL12A, Il12B, Il6 and IL-12p70 that help produce the particular T cell differentiation.
Fucose Sugars Also Trigger T2 and Tph
 When the sugar fucose is recognized on microbes, T2 and Tph are triggered, which starts immune responses. These processes alter the RAF1 signalosome and create a different one called a fucose signalosome. H. pylori, Schistosoma and other dangerous microbles trigger this signalosome. Through very complex pathways involving BCL-3-p50, genetic networks are altered. Specific promoters and inhibitors of genes are altered and there is a shift from the T1 to the T2 programs. IL-27 is involved as is the cytokine IFNβ.
When the sugar fucose is recognized on microbes, T2 and Tph are triggered, which starts immune responses. These processes alter the RAF1 signalosome and create a different one called a fucose signalosome. H. pylori, Schistosoma and other dangerous microbles trigger this signalosome. Through very complex pathways involving BCL-3-p50, genetic networks are altered. Specific promoters and inhibitors of genes are altered and there is a shift from the T1 to the T2 programs. IL-27 is involved as is the cytokine IFNβ.
The fucose pathway is also involved in immunity and tolerance. This occurs through action of macrophages and IL-10.
Dectin 2
 Another CLR receptor is D2 that helps with the responses related to T17 and T2. This receptor operates without the ability to send its own signals. Instead it works with another adaptor molecule called the called the Fc receptor common γ-chain (FcRγ). Dectin 2 receptor is on macrophages, dendritic cells and other immune cells. It responds to particular mannose structures that are part of fungi and mycobacteria. It responds to aspergillus and house dust mites Dermatophagoides. It works with the cytokine NF-κB, which induces many others including IL-6, IL-23, IL-12 and IL-1β.
Another CLR receptor is D2 that helps with the responses related to T17 and T2. This receptor operates without the ability to send its own signals. Instead it works with another adaptor molecule called the called the Fc receptor common γ-chain (FcRγ). Dectin 2 receptor is on macrophages, dendritic cells and other immune cells. It responds to particular mannose structures that are part of fungi and mycobacteria. It responds to aspergillus and house dust mites Dermatophagoides. It works with the cytokine NF-κB, which induces many others including IL-6, IL-23, IL-12 and IL-1β.
Dectin 2 induces cytokines that can both increase inflammation and decrease it. Pathways include pro inflammatory tumor necrosis factor (TNF), IL-6 and IL-23, and anti-inflammatory IL-10 and IL-2. It is part of many complex processes such as making a caspase inflammasome. In one pathway it indirectly increases dectin 1 pathways against fungi. It pushes the pathways towards T2 response from T1. Dectin 2 responses appear to be very specific to the offending microbe. Most of the time it works with other receptors and can’t trigger the pathways by itself.
MINCLE with T1 and T17
 Another CLR receptor with a very strange name is MINCLE (or Macrophage-inducible C-type lectin receptor also known as CLEC4E) has been found to be involved in cell death. It can stop responses to cancer development such as helping pancreatic cancer. But, it is involved with many mechanisms and participates in the positive response to fungi and tuberculosis.
Another CLR receptor with a very strange name is MINCLE (or Macrophage-inducible C-type lectin receptor also known as CLEC4E) has been found to be involved in cell death. It can stop responses to cancer development such as helping pancreatic cancer. But, it is involved with many mechanisms and participates in the positive response to fungi and tuberculosis.
MINCLE responds to some particular mannose structures. It works both independently and with other CLRs. It is found on dendritic cells, macrophages and leukocytes. There are many different responses that can occur, and the reasons for each are not yet clear. It can produce T1 immunity that is protective with help from the Toll like pattern receptors TLR2, TLR4 an TLR7. This produces very strong cytokine responses. It can also skew towards T2, which can hurt the defense against fungus. This works through several complex inflammasomes. A totally different response produces T17 affecting many different cytokines. It appears that the different co receptors form relationships that trigger these different responses.
Different Receptor Scaffolds
 As mentioned, various receptors can work together to produce very different results. Dectin 1 and 2 and MINCLE have very different responses. The pathways involved are just now being worked out and involve many other obscure molecules that have not been mentioned already. It appears that these various pathways form machines called inflammasomes and signalosomes that direct different effects.
As mentioned, various receptors can work together to produce very different results. Dectin 1 and 2 and MINCLE have very different responses. The pathways involved are just now being worked out and involve many other obscure molecules that have not been mentioned already. It appears that these various pathways form machines called inflammasomes and signalosomes that direct different effects.
 There are also other receptors that have not been mentioned yet. Some produce adaptive immunity to particular microbes, but the mechanisms are not yet clear. These are all involved in producing very particular T cells that use even more specific multiple cytokines and pathways. CLRs are now very relevant to creating specific vaccines for many microbes, including HIV and Ebola.
There are also other receptors that have not been mentioned yet. Some produce adaptive immunity to particular microbes, but the mechanisms are not yet clear. These are all involved in producing very particular T cells that use even more specific multiple cytokines and pathways. CLRs are now very relevant to creating specific vaccines for many microbes, including HIV and Ebola.
Part of the reason that there are so many different responses to these receptors is that there are also many different kinds of dendritic cells. Very specific subsets of macrophages and dendritic cells have different responses as well as different types of molecular triggers. This makes producing vaccines much more difficult. Research is now ongoing to find more general stimuli that would affect many cell types. These might include β-glucan carbohydrate particles from the microbes that might only produce predictable T1 and T17 T cells. For viruses multiple CLRs might be used at the same time in a vaccine. If only one carbohydrate is chosen, then multiple effects could occur. Tumors often produce fucose molecules and this might be able to be used as a vaccine for cancers.
CLRs are unique in producing so many different responses from T helper cells. This does not occur with most other pattern recognition receptors.
Producing Many Kinds of T Helper Cells
 The very complex lifestyle of T cells appears to be even more complex than previously thought. Among its many talents, creating so many very particular T cells is quite remarkable. Special T cells damp down specific killer cells before they cause damage to human tissues. Helper cells are vital for protecting against auto immune responses from many types of microbes. They orchestrate unique responses to each situation.
The very complex lifestyle of T cells appears to be even more complex than previously thought. Among its many talents, creating so many very particular T cells is quite remarkable. Special T cells damp down specific killer cells before they cause damage to human tissues. Helper cells are vital for protecting against auto immune responses from many types of microbes. They orchestrate unique responses to each situation.
It was known that dendritic cells and T cells communicate to make the vital decisions about when a T cell activates. Now, the latest research is uncovering a wide variety of receptors and signals on dendritic cells that help to determine which type of T cells are produced by differentiation. What is striking about this is that with many aspects of cellular responses, there are many possible different interacting pathways that operate and can produce a wide range of varied responses, even for the same signals.
Communication between subsets of dendritic cells and the many T cells is much more complex than previously understood. Elaborate signaling of T cells is just beginning to be understood.