 Immune cells in the gut are produced in response to environmental signals and communication between many different types of cells. The gut is a very special environment that includes cooperative and competitive efforts between many cells including trillions of friendly and unfriendly microbes, intestinal epithelial cells, and immune and blood cells. The gut lining cells must determine which microbes are friendly and which must be kept away. All nutrition is determined by cooperation of microbes that digest and epithelial cells that take in food particles. A process is necessary where special T cells inhibit immune reactions to food.
Immune cells in the gut are produced in response to environmental signals and communication between many different types of cells. The gut is a very special environment that includes cooperative and competitive efforts between many cells including trillions of friendly and unfriendly microbes, intestinal epithelial cells, and immune and blood cells. The gut lining cells must determine which microbes are friendly and which must be kept away. All nutrition is determined by cooperation of microbes that digest and epithelial cells that take in food particles. A process is necessary where special T cells inhibit immune reactions to food.
Food is a “foreign” substance and T cells are designed to fight against invading foreign substances including toxins and microbes. Special T cells must suppress the reactions that would normally occur from a large number of immune and blood cells to fight invading foreign food particles. Without these very particular T regulatory cells there would be a vast increase in autoimmune diseases. Production of these vital T cells occurs because of many different signals.
A previous post introduced the topic of food signals influencing the development of immunity. It described aryl hydrocarbons, vitamins A and D as special signals related to different immune cell production. This post will describe the vital signals necessary to produce these T regulatory cells including the well-known vitamins and metabolites folic acid, niacin, vitamin A, vitamin D3, tryptophan, short chain fatty acids (SCFAs), as well as many cytokines.
Unique Need for T cells in Gut
 T cells throughout the body defend against invasion by microbes and toxic chemicals. They respond to molecules that should not be in the body when they trigger receptors for a particular molecular pattern. Anything that is not recognized as normally part of “self” becomes a target for the T cell.
T cells throughout the body defend against invasion by microbes and toxic chemicals. They respond to molecules that should not be in the body when they trigger receptors for a particular molecular pattern. Anything that is not recognized as normally part of “self” becomes a target for the T cell.
However, T cells in the gut have to deal with a vast array of new chemicals in form of foods and the largest amount of microbes. Receptors and signals on gut T cells are different from any other. They suppress immune reactions to novel but not dangerous molecules. Special gut regulatory T cells are specially educated to avoid dangerous reactions to food. They must be educated and maintained by elaborate signaling from many sources. These gut special regulatory T cells communicate widely with other T cells. It is signaling from the local environment that controls their complex behavior. Microbes provide a constant source of small molecules that regulate and modulate gut T cell behavior.
At the gut mucosa, many unique immune cells work together to defend aggressive microbes. These unique immune cells include IgA-secreting plasma cells, γδ T cells, innate lymphoid cells (ILCs) and T helper 17 cells (TH17 cells). With vigorous behavior from these cells, reactions to harmless molecules must be inhibited. Inflammation of the intestines frequently occurs through reactions to food and microbes that are not aggressive. These inflammatory bowel diseases are caused by reactions from effector T cells and myeloid white blood cells and can damage tissues.
Regulating these complex immune responses falls to a particular T cell that has a unique mechanism. It also has a very strange long name—box P3 (FOXP3)-expressing CD4+ regulatory T (Treg). For this article, we will call them special T cells or FOXP3 for short.
Previous studies of autoimmunity show that missing sub populations of T cells causes disease in many organs including the gut. These studies focused on CD4+CD25 cells. Recent research related to food reactions finds that this is not the way food reactions are suppressed. Recent research shows that FOXP3 is much more relevant.
In fact, mutations of FOXP3 caused inflammation in many organs and without this special T cell, colitis occurs. Mutations cause many diseases in endocrine tissues and general dys regulation of immunity. Antibodies against T cells in cancer treatment also cause colitis. Multiple different versions of FOXP3 affect critical cytokines such as IL-10. These subsets work together with the FOXP3+ regulatory T cell to maintain healthy tissues.
An important sub category of T cells is CD4+ and 10% of these are the special regulatory T cell that stops reactions from food. They exist in all tissues but much higher amounts (30%) in the lamina of the intestinal lining.
With signals, FOXP3 cells regulate many different cells and pathways. They secrete multiple cytokines that regulate other cellular reactions related to food materials. These inhibitory signals have to maintain tolerance to foods and friendly microbes. A particular subset needs to control the roles of other cells that train T cells to attack in the lymph education centers. The number of these educational T cells is regulated by signals from microbes as well.
Sub Types of FOXP3
 There are two lines of FOXP3+ Treg cells. Each produces multiple different kinds based on the types of receptors they have and what cytokines and other signals they can produce. One comes from the thymus and the other is trained in the local gut region. Locally born T cells are more important and necessary. Training of these T cells is from epithelial cell signals and microbes about the particular foods being ingested. Specific protein transcription factors are necessary to produce these regulatory T cells. Many complex receptors and pathways are involved in preparing the T cell for the complexity of not over reacting to food.
There are two lines of FOXP3+ Treg cells. Each produces multiple different kinds based on the types of receptors they have and what cytokines and other signals they can produce. One comes from the thymus and the other is trained in the local gut region. Locally born T cells are more important and necessary. Training of these T cells is from epithelial cell signals and microbes about the particular foods being ingested. Specific protein transcription factors are necessary to produce these regulatory T cells. Many complex receptors and pathways are involved in preparing the T cell for the complexity of not over reacting to food.
Thymus derived special gut regulatory T cells respond to many traditional receptors for T cells. These receptors differentiate “self” cells from “foreign” signals. The cytokine IL-2 is an important signal in this process.
Signals stimulate genetic networks by a promoter of the same name (forkhead box P3 promoter) inducing the Foxp3 gene. In the intestine, T cell training starts with signals and receptors related to microbe material. Other special signals are involved including transforming growth factor‐β (TGFβ) and retinoic acid‐mediated signals (related to vitamin A in food). Mechanisms include methyl tags removed in the genetic network and butyrate from microbes that remodels histones with acetyl groups.
Many Different Signals and T cell Subtypes
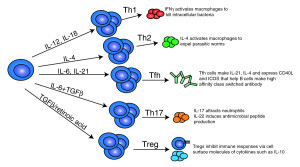 In the colon there are vast amounts of different signals, which cause alterations in T cells. In the thymus other signals prepare new T cells. They are further modified when they meet local intestinal signals. Many very elaborate signaling pathways occur that stimulate more of these special cells. Some special T regulatory cells are produced during early life with introduction of food and then are continuously reproduced. Reproduction is stimulated by the cytokine IL-2.
In the colon there are vast amounts of different signals, which cause alterations in T cells. In the thymus other signals prepare new T cells. They are further modified when they meet local intestinal signals. Many very elaborate signaling pathways occur that stimulate more of these special cells. Some special T regulatory cells are produced during early life with introduction of food and then are continuously reproduced. Reproduction is stimulated by the cytokine IL-2.
A recent finding is that when T cells are trained in the intestine to avoid food reactions, some travel to the thymus and share the receptors and responses with them. Early on, cells from the thymus travel to the intestine and stay in a small niche. With diminished signals from microbes and less intestinal trained T cells, this niche is stimulated by signals and grows to help. It is hard to follow individual cells and the many ways that these two bodies of cells interact is not clear. But, the impact of constant signals from microbes is very important.
There are, in fact, more sub types of FOXP3 cells and some don’t produce the necessary inhibition of inflammation. These sub populations can produce cytokines that cause inflammation. Some of these cells start cooperating with the suppression related to food and then are altered for particular purposes.
Multiple Genes Interact with FOXP3 to Protect Against Food Reactions
 It is now clear that particular epigenetic changes occur in T cells that maintain their role in protecting inappropriate reactions to food. Two gene locations other than FOXP3 need to have methyl groups removed for the T cell to be stable as a protector of food reactions.
It is now clear that particular epigenetic changes occur in T cells that maintain their role in protecting inappropriate reactions to food. Two gene locations other than FOXP3 need to have methyl groups removed for the T cell to be stable as a protector of food reactions.
 Interactions between these two gene regions and FOXP3 determines subtypes. Taking the methyl group off of one of the gene regions (CNS2) allows it to interact with FOXP3. It needs a particular protein transcription factor as well. Cytokine IL-2 helps this process. It is not clear what triggers demethylation. Antigens from food, signals from microbes and IL-2 maintain the state of demethylation.
Interactions between these two gene regions and FOXP3 determines subtypes. Taking the methyl group off of one of the gene regions (CNS2) allows it to interact with FOXP3. It needs a particular protein transcription factor as well. Cytokine IL-2 helps this process. It is not clear what triggers demethylation. Antigens from food, signals from microbes and IL-2 maintain the state of demethylation.
Specific environments alter regulatory T cells. These have particular unique transcription factors that alter genetic networks and send particular signals that suppress other immune cells. One variation occurs with tuberculosis infection in the lungs. This type of inflammation stimulates different versions of these T regulatory cells. Another is different during a particular type of intestinal inflammation (after bone marrow transplantation). Studies show that these subtypes work with FOXP3 to maintain liver, lung and intestines. FOXP3 learns from these subtypes that are responding to particular unique types of infection.
FOXP3 gathers information and techniques from travel to other tissues and the experience of their unique inflammation. FOXP3 uses these techniques to influence more cells to cooperate in their food reactions.
Three populations of FOXP3
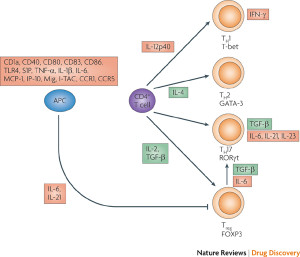
Three large populations of FOXP3 T reg cells are determined by particular receptors and pathways.
- RAR‐related orphan receptor γt (RORγt) – T cells of this type are called RORγt+ Treg cells.
- GATA-binding protein 3 (GATA3)
- Helios
- Neuropilin 1 (NRP1)
- RAR uses a particular transcription factor (STAT3) from the cytokine signal Il-6 and IL-2. These suppress many different other T cells from responding. 65% of colon and 35% T reg cells are positive for RAR.
- One subtype has GATA and Helios and can be stimulated by fatty acids.
- NRP1− Treg cells are 40% of small intestine cells lamina. They are critical to all types of food reactions.
 GATA3+ Treg cells are related to cytokine IL-33, which produces rapid respose to inflammation from food. It increases FOXP3 and helps maintain order. Most GATA are also Helios and don’t require constant signals from microbes, probably trained in the thymus. As more microbes signals trigger more GATA and IL-2, there are more GATA cells produced.
GATA3+ Treg cells are related to cytokine IL-33, which produces rapid respose to inflammation from food. It increases FOXP3 and helps maintain order. Most GATA are also Helios and don’t require constant signals from microbes, probably trained in the thymus. As more microbes signals trigger more GATA and IL-2, there are more GATA cells produced.- Without GATA, it is hard for FOXP3 cells to accumulate in the midst of inflammation. A great amount of IL-33 signals are sent from epithelial gut lining cells during inflammation. They produce less normally. IL-33 with IL-2 and special T receptors activates GATA3 greatly increasing FOXP3. A special T cell receptor OX40 is very helpful in this situation.
These three sub populations in fact work together and are necessary to the very complex response to the millions of different food molecules.
FOXP3 T cells Become Mature
 T regulatory cells can mature and become very powerful at suppressing food reactions. These develop a wide range of new receptors. They need constant signals about food to remain in this state.
T regulatory cells can mature and become very powerful at suppressing food reactions. These develop a wide range of new receptors. They need constant signals about food to remain in this state.
IL-10 is vital to the entire process of maintaining FOXP3 and suppressing food reactions. Mature cells trigger IL-10. Helios types are major producers of IL-10 in the colon. Without this constant IL-10, people get spontaneous inflammation of the colon (colitis). IL-10 suppresses blood cell activity including many other T helper cells. IL-10 is regulated by STAT3 and not by FOXP3, which stimulates only a small number of genes. The main effect of FOXP3 is to stop activating specific genes. STAT3 is stimulated by microbe signals to make IL-10 from T cells. T cells then suppress microbe signals and effects. In fact, it is more complex than this and multiple pathways lead to IL-10.
Without T cell receptors to IL-10, severe colitis develops. In this case, some other cells such as B-lymphocytes and macrophages make a smaller amount of IL-10.
Maintaining FOXP3 Needs Constant Signals
 Making local special T cells needs signaling from food particles, epithelial intestinal cells, macrophages, dendritic cells and innate lymphoid cells. These are stimulated by microbe signals and they then send signals to train T cells.
Making local special T cells needs signaling from food particles, epithelial intestinal cells, macrophages, dendritic cells and innate lymphoid cells. These are stimulated by microbe signals and they then send signals to train T cells.
Vitamin A is a major signal. Microbes stimulate epithelial cells for several pathways (IDO and TSLP WNT) that produce dendritic cell enzymes required to turn Vitamin A into retinoic acid. Microbes stimulate macrophages to make IL‐1β cytokine, which stimulates subtypes of T cells ROR.
Clostridia microbes eat fiber and produce short chain fatty acids (SCFA), another very important signal. SCFAs filter through epithelium to immune cells and trigger T cells and dendritic cells directly. Through complex pathways, they affect histones stimulating more T cells. Bacteroides fragilis send vesicles that activate IL-10. IL-10 affects T cells with the special IL-10 receptor. This stimulates STAT and more IL-10, which suppresses many different immune cells.
Microbe Signals for FOXP3
 Gut microbes increase numbers of FOXP3 T cells and alter their functions. Particular strains of Clostridia induce more T cells strongly in the colon. They specially make ROR types without Helios, not GATA3 types. These can also trigger IL-10. They also trigger immune cells that make IL-22. IL-22 makes the epithelium stronger, which stops food particles from entering. This also decreases antigens from microbes entering and increases diversity among Clostridia. It stops large-scale stimulation of T cells. Other groups of microbes have other effects such as five microbes working together increasing the amount of T cells.
Gut microbes increase numbers of FOXP3 T cells and alter their functions. Particular strains of Clostridia induce more T cells strongly in the colon. They specially make ROR types without Helios, not GATA3 types. These can also trigger IL-10. They also trigger immune cells that make IL-22. IL-22 makes the epithelium stronger, which stops food particles from entering. This also decreases antigens from microbes entering and increases diversity among Clostridia. It stops large-scale stimulation of T cells. Other groups of microbes have other effects such as five microbes working together increasing the amount of T cells.
Clostridia lives at the mucous layer near epithelium and have strong effects. They increase a number of critical cytokines. Some of the effect is through making short chain fatty acids that affect dendritic and T cells.
Lactobacilli signals also increase T cells. Bacteroides fragilis signals makes T cells mature and increases IL-10. Bacterial polysaccharide is a signal sent in vesicles.
Diet Signals
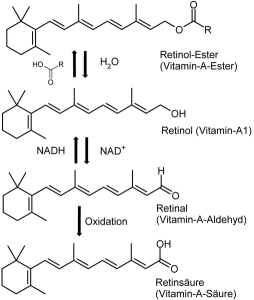
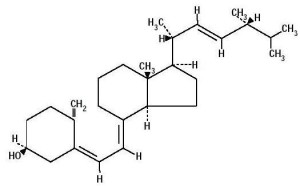 Vitamin A is a critical signal for production of FOXP3. It is metabolized to retinoic acid by dendritic cells at the lamina. It is vital to produce more special T cells along with cytokine TGFβ. It stimulates multiple pathways and cytokines that are related to alleviating inappropriate responses to food by the immune system. When it encounters other cytokines such as IL-15, it can signal inflammation. It is known to be a critical switch.
Vitamin A is a critical signal for production of FOXP3. It is metabolized to retinoic acid by dendritic cells at the lamina. It is vital to produce more special T cells along with cytokine TGFβ. It stimulates multiple pathways and cytokines that are related to alleviating inappropriate responses to food by the immune system. When it encounters other cytokines such as IL-15, it can signal inflammation. It is known to be a critical switch.
Another vital signal is vitamin D3. This metabolizes to 1,25 dihydroxyvitamin D3, which stimulates receptors for more production of FOXP3.
Folic acid (B9) is a strong signal from diet and microbes. A receptor for folic acid in T reg cells helps their survival through a BCL-2 pathway. With less folic acid there are less T reg cells.
Tryptophan is an amino acid signal in food metabolized to kynurenin in epithelial cells and immune dendritic cells. Tryptophan stimulates T cells through several mechanisms. Kynurenin is altered to kynurenic acid, which triggers other receptors related to inflammation in the bowel. Another metabolite of tryptophan is the vitamin niacin (B3), which triggers macrophages in the colon inducing more T reg cells. It is also related to colitis.
 Fibers are large complex carbohydrate signals. Fibers are metabolized (fermented) by microbes, especially Clostridia and Bacteroides. Products of this fermentation include acetate, butyrate and propionate. Many genes in Clostridia produce short chain fatty acids (SCFAs). They stimulate FOXB3 cells, epithelial cells and immune dendritic cells. They alter enzymes that place acetyl tags on histones and trigger other signals. They suppress inflammatory cytokines by histone modifications that make more T cells.
Fibers are large complex carbohydrate signals. Fibers are metabolized (fermented) by microbes, especially Clostridia and Bacteroides. Products of this fermentation include acetate, butyrate and propionate. Many genes in Clostridia produce short chain fatty acids (SCFAs). They stimulate FOXB3 cells, epithelial cells and immune dendritic cells. They alter enzymes that place acetyl tags on histones and trigger other signals. They suppress inflammatory cytokines by histone modifications that make more T cells.
Butyrate signals start inflammation through genes in dendritic cells. But, these trigger more FOXB3 and IL-10. SCFAs also directly trigger more T reg cells by other histone affects. The histone of FOXP3 is involved. They produce more T reg cells by many different mechanisms.
Immune Cell Signals
 Dendritic cells at the lamina can extend arms through the epithelial layer and grab antigens and signals from microbes. They also receive these through a transport system with Goblet cells. Vesicles from microbes go directly to these dendritic cells. These cause many pathways including the vitamin A pathway. They produce more T reg cells.
Dendritic cells at the lamina can extend arms through the epithelial layer and grab antigens and signals from microbes. They also receive these through a transport system with Goblet cells. Vesicles from microbes go directly to these dendritic cells. These cause many pathways including the vitamin A pathway. They produce more T reg cells.
Dendritic cells are necessary signaling partners to produce more FOXP3 cells. Many complex factors and pathways increase FOXP3 production. Each sub type of T cells interacts constantly with dendritic cells. ROR affects how dendritic cells function, which in turn signals more FOXP3.
Complex environmental situations alter signals from macrophages, lymphocytes and dendritic cells. They are able to respond to the different types of microbes as well as different diets to regulate the amount of T cells needed to maintain order. Signals from the lymph centers in the gut just under the lining send many different kinds of signals that produce the exact sub types of T cells, stabilize them with constant support signals, and help maturation for strong cells.
Special T cells Inhibit Immune Reactions to Food
 Special varieties of T cells must be produced to avoid constant immune reactions to the millions of different “foreign” food particles we ingest each day. Without FOXP3 T cells most people would have dangerous reactions to food particles.
Special varieties of T cells must be produced to avoid constant immune reactions to the millions of different “foreign” food particles we ingest each day. Without FOXP3 T cells most people would have dangerous reactions to food particles.
But, these regulatory T cells must be stimulated by a wide range of signals and constantly reinforced and maintained. These signals come from food particles, epithelial intestinal cells, immune cells, blood cells and microbes. Recent research shows that many well known vitamins and food molecules have vital roles in stimulating these vital FOXP3 T cells. This research demonstrates just how complex cellular signaling is related to immune function.
The science of what microbes help and hurt human diseases is in its infancy. Fecal transplants thus far helped Clostridium difficile infection, but have not helped inflammatory bowel disease. The very specific ways that bacteria interact with T cells will be critical to figure out how alterations in microbes in the gut can help immune diseases such as asthma, multiple sclerosis and inflammatory bowel disease.
While immune signals and pathways are vastly complex, it is a very exciting field of research. By communicating as a group, these cells are making vital decisions that affect the entire organism.
 GATA3+ Treg cells are related to cytokine IL-33, which produces rapid respose to inflammation from food. It increases FOXP3 and helps maintain order. Most GATA are also Helios and don’t require constant signals from microbes, probably trained in the thymus. As more microbes signals trigger more GATA and IL-2, there are more GATA cells produced.
GATA3+ Treg cells are related to cytokine IL-33, which produces rapid respose to inflammation from food. It increases FOXP3 and helps maintain order. Most GATA are also Helios and don’t require constant signals from microbes, probably trained in the thymus. As more microbes signals trigger more GATA and IL-2, there are more GATA cells produced.