 Microbes show advanced cognitive abilities – communication, group activity, and complex decision making. But, what can we say when a remarkable microbe makes stem cells?
Microbes show advanced cognitive abilities – communication, group activity, and complex decision making. But, what can we say when a remarkable microbe makes stem cells?
How can the leprosy bacteria, mycobacterium leprae, turn a Schwann cell into a stem cell?
Leprae’s captive vehicle, the stem cell, then becomes different types of cells to travel to different destinations.
Are Leprae, like viruses and mitochondria, more intelligent because they give up DNA functions and travel with less weight?
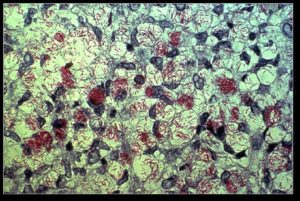
Leprosy, or Hanson’s disease, has, most often, been caused by a bacteria mycobacterium leprae. Recently. a relative species called mycobacterium lepromatosis was also found to cause leprosy. 200,000 people are diagnosed with leprosy each year.
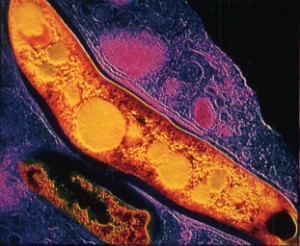 Mycobacteria are most well known for another related species, mycobacterium tuberculosis, which has a similar appearance to leprae. These two probably evolved from the same bacterial line, but the leprae gradually lost more and more of its genes, making it very dependent upon other cells for its functions. The lost functions include most of the ability to use oxygen, anaerobic respiration, and other important metabolic processes.
Mycobacteria are most well known for another related species, mycobacterium tuberculosis, which has a similar appearance to leprae. These two probably evolved from the same bacterial line, but the leprae gradually lost more and more of its genes, making it very dependent upon other cells for its functions. The lost functions include most of the ability to use oxygen, anaerobic respiration, and other important metabolic processes.
A previous post noted that viruses might also have been bacteria that lost functions. And 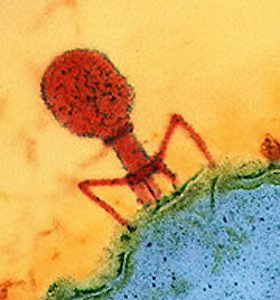 that losing functions may not be a sign of weakness or less capability, but rather a sign of increased intelligence, transferring whatever baggage it can to others in order to move and evolve more rapidly. The mitochondria, critical to all human cell’s processes, functions very well inside of the large neuron.
that losing functions may not be a sign of weakness or less capability, but rather a sign of increased intelligence, transferring whatever baggage it can to others in order to move and evolve more rapidly. The mitochondria, critical to all human cell’s processes, functions very well inside of the large neuron.
One study found that the populations of bacteria causing tuberculosis and leprosy at one time were equal. This study shows that leprosy then lost 1200 protein coding genes, mostly by recombination.
Cannot Culture Leprae
Because half of leprae’s genome has become pseudo genes, the leprosy bacteria must live and reproduce in other cells. Being only able to live as an intracellular  parasite makes it extremely difficult to study. Without the means for independent survival, researchers cannot culture the bacteria in a lab. The only way it can be studied is by growing it in animals, especially mice and armadillos. Even staining it is difficult in animal tissues, the process needing to use carbol fuchsin stain. Even then, it takes weeks to see the stained bacteria. For all of these reasons there is no test to know when someone has the disease.
parasite makes it extremely difficult to study. Without the means for independent survival, researchers cannot culture the bacteria in a lab. The only way it can be studied is by growing it in animals, especially mice and armadillos. Even staining it is difficult in animal tissues, the process needing to use carbol fuchsin stain. Even then, it takes weeks to see the stained bacteria. For all of these reasons there is no test to know when someone has the disease.
Mycobacterium leprae is also one of the slowest growing bacteria, having the longest doubling time of all known bacteria. One reason for the very slow rate of growth in mycobacterium is a very complex cell wall with a thick waxy coating making both leprosy and tuberculosis difficult to destroy.
Living Inside A Macrophage
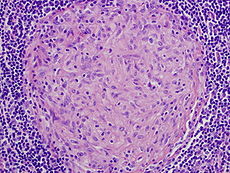
One of the features of mycobacteria infection is the granuloma.
Granuloma are a special form of infection, where the immune system attempts to wall off bacteria, a dense circular structure stuffed with macrophages, large white blood cells eating bacteria. However, the leprosy bacteria are extremely clever and somehow are able to live inside the macrophage in the granuloma. In fact, leprosy takes over control of the macrophage and uses it to travel to other parts of the body. This is particularly  remarkable since the macrophage is the cell that destroys bacteria.
remarkable since the macrophage is the cell that destroys bacteria.
Leprosy primarily forms granulomas in nerves outside the brain and in the respiratory tracts. Externally there are skin lesions. The disease causes tissue loss with deformed fingers and toes. Also unknown is why 95% of human beings are immune to leprosy.
Since there is no test, it is only noticed when symptoms occur. Symptoms include deforming lesions and loss of sensation in hands and feet. It also causes muscle weakness.
There has been little known about how it spreads, except that it seems to especially like to live in Schwann cells in the nervous system.
Leprae Likes Schwann Cells
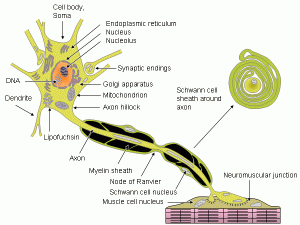 Schwann cells are similar to glia in the brain, but live in the peripheral nervous system supporting neurons by making myelin (in the brain oligodendrocytes perform this function). It is not widely known that Schwann cells perform many more functions for neurons, other than just making myelin. They are critical support cells for all neurons, not just the ones that use myelin to increase the speed of signal transmission. For example, they provide extracellular matrix to maintain synapses, nutrients and special factors to allow neurons to grow. They are critical in fetal development and in regeneration of damaged neurons.
Schwann cells are similar to glia in the brain, but live in the peripheral nervous system supporting neurons by making myelin (in the brain oligodendrocytes perform this function). It is not widely known that Schwann cells perform many more functions for neurons, other than just making myelin. They are critical support cells for all neurons, not just the ones that use myelin to increase the speed of signal transmission. For example, they provide extracellular matrix to maintain synapses, nutrients and special factors to allow neurons to grow. They are critical in fetal development and in regeneration of damaged neurons.
 But, the most widely known function is to provide the myelin sheath, insulation for the axon, which allows the electrical signal to be sent much more rapidly in long important nerves. The nerve from the spinal cord to the legs can be three feet in length, with approximately 10,000 different Schwann cells covering this length. But, each Schwann cell is attached to only one axon.
But, the most widely known function is to provide the myelin sheath, insulation for the axon, which allows the electrical signal to be sent much more rapidly in long important nerves. The nerve from the spinal cord to the legs can be three feet in length, with approximately 10,000 different Schwann cells covering this length. But, each Schwann cell is attached to only one axon.
The myelin sheath consists of mainly a large number of membranes, formed by the cell wrapping around the axon many times, as many as 100, and then secreting myelin in between each layer.
Leprae Make Human Stem Cells
Since it has been so difficult to find out how leprae enters and travels in human beings, it was shocking to learn that it can turn a Schwann cell into a stem cell.
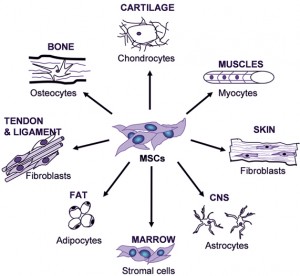 Leprosy bacteria with a very small amount of DNA are able to change the genes of the Schwann cell into an earlier type of cell that had previously transformed into Schwann cells. The stem cells that they become (MSCs) are similar to those in bone marrow, which can become bone, connective tissue, muscles and nerves.
Leprosy bacteria with a very small amount of DNA are able to change the genes of the Schwann cell into an earlier type of cell that had previously transformed into Schwann cells. The stem cells that they become (MSCs) are similar to those in bone marrow, which can become bone, connective tissue, muscles and nerves.
How leprosy first attacks the nervous system is still unknown. But, it is known that, as well as hiding in macrophages in granuloma, they hide in Schwann cells where immune cells cannot find them. When the Schwann cell is triggered to change cell type, it becomes a pluripotent stem cell.
Surprisingly, these stem cells, with Leprae inside, became muscle cells and were observed migrating to muscle tissue. Leprae uses this stem cell trick to infiltrate both muscle and nerves.
Induced Stem Cells
The word pluripotent refers to the fact that the cell can divide into a variety of different lines of cells. The different lines of cells are originally determined in the fetus. The original embryonic stem cells can divide into any line of cells.
Pluripotent cells that can be induced from other cells, such as skin cells, are called induced pluripotent stem cells or iPSC, and can turn into many different types of cells but not all the types. The mesenchyme is one type of cell line in the fetus that makes connective tissue, bone, and cartilage. The iPSC that are made from leprae is in the mesenchymal line of cells, called MSC (picture above).
 Researchers have been attempting to find ways to make iPSC cells, to avoid using fetal stem cells in therapies. The first major breakthrough in 2006 inducing pluripotent stem cells used retroviruses to bring four key genes into a skin cell to reprogram it.
Researchers have been attempting to find ways to make iPSC cells, to avoid using fetal stem cells in therapies. The first major breakthrough in 2006 inducing pluripotent stem cells used retroviruses to bring four key genes into a skin cell to reprogram it.
These first four genes were: Oct-3/4, SOX2, c-Myc, and Klf4.
Two of these four genes c-Myc, and Klf4 can cause a switch to create cancer cells at a rate of about 20%. Without using the cancer causing genes the process was much less efficient.
Later, another gene, Nanog, was used instead of the cancer causing genes.
Fibroblasts were transformed into stem cells using a different set of genes : Oct4, SOX2, Nanog and a new gene LIN28.
Even more recently, microRNA strands have been carried by a virus to reprogram stem cells.
There are still major problems with these methods. As well as stimulating cancer these methods are still very inefficient and the viral insertion introduces errors. Other trials have included jumping genes, plasmids and adenovirus, which does not actually insert material into the chromosome but just uses the machinery of the cell.
Although still inefficient at this time, these methods are able to produce many different cells including neurons, muscle and heart cells.
Leprae Makes a Stem Cell
Very surprisingly, the leprae bacteria is able to rework the cells internal mechanisms to turn them into a stem cell. The resultant cell is in the stage of progenitor/stem-like cells known as pSLC.
The leprae process alters sets of genes that decrease activity maintaining the Schwann cell, and increases the activity related to the mesoderm. When the Schwann cell is reprogrammed it stops making myelin causing neurological damage.
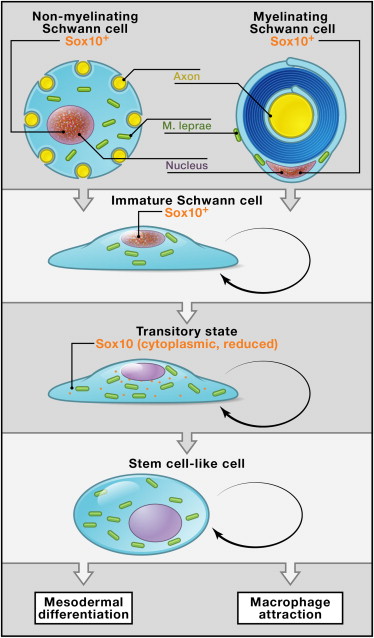
Specifically, leprae infected Schwann cells decrease the function of genes in the Schwann cell line including genes: p75, Sox10, ErbB3, L1Cam, and also the myelin gene Mpz.
The stimulated genes include those for development of skeletal muscles and smooth muscles through epigenetic effects on chromatin structures. These special genes form muscle. Genes that leprae use to stimulate mesoderm include: Nap1l1, H2afz, Hells, and Ezh2, as well as Wnt and Notch genes.
Despite these dramatic changes, the new cells do not become cancerous.
Another Good Trick
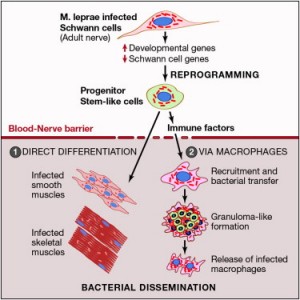 These mesenchyme cells also can become a granuloma crowded with immune white blood cell macrophages filled with the leprosy bacteria.
These mesenchyme cells also can become a granuloma crowded with immune white blood cell macrophages filled with the leprosy bacteria.
In order to build these granulomas, Leprae stimulates immune related chemokine cytokine growth factor genes with epigenetic mechanisms to secrete substances that attract immune cells. The immune cells eat or engulf the bacteria and leprosy is able to live in the immune cell and modulate it to spread the infection. The infected immune cells migrate and have the ability to change cells types.
Viruses, Mitochondria and Leprae Travel Lite
In a previous post, it was noted that viruses might well have been microbes that evolved by losing much of the DNA, just as the leprosy bacteria has done. Despite the fact that a virus is totally dependent upon other cells for its metabolism, and reproduction, it has an independent life and shows group activity and surprising ingenuity. Viruses are master manipulators of cells, as are leprae.
 Another microbe that lost some of its genes is the mitochondria, which is vital to our human cells producing energy wherever it is needed. A previous post described how mitochondria travel throughout the huge neuron and multiply when necessary to provide energy for any necessary project, such as repairing an axon or building new dendrites. They also monitor, repair or eliminate damaged mitochondria. While dependent upon the human cells for much of their function, they maintain independent genes to produce energy and to reproduce. They appear to have a vital semi-autonomous existence within the cells providing critical energy whenever it is needed. These cells might also not be so successful unless they had given up carrying unnecessary genes that the large cell can manage.
Another microbe that lost some of its genes is the mitochondria, which is vital to our human cells producing energy wherever it is needed. A previous post described how mitochondria travel throughout the huge neuron and multiply when necessary to provide energy for any necessary project, such as repairing an axon or building new dendrites. They also monitor, repair or eliminate damaged mitochondria. While dependent upon the human cells for much of their function, they maintain independent genes to produce energy and to reproduce. They appear to have a vital semi-autonomous existence within the cells providing critical energy whenever it is needed. These cells might also not be so successful unless they had given up carrying unnecessary genes that the large cell can manage.
One of the lessons of microbe social life is that bacteria will always travel as lite as possible. By shedding functions to other bacteria and cells they  develop symbiotic relationships that have been a staple of evolution. Recent research showed that microbes can be quite selfish in this regard, letting other members of the colony deal with antibiotic resistance and other issues, saving energy for themselves.
develop symbiotic relationships that have been a staple of evolution. Recent research showed that microbes can be quite selfish in this regard, letting other members of the colony deal with antibiotic resistance and other issues, saving energy for themselves.
But, these smaller leaner creatures appear to retain the intelligence of larger microbes. Is it possible that smaller, leaner bacteria, mitochondria, and viruses are even more intelligent than other microbes because they have pared themselves down to the smallest amount of luggage?
Are viruses, and leprae, also somewhat smarter and more nimble because they don’t have worry about so much baggage?