 The nervous system regulates the heart, the lungs, and the GI tract often through circuits that rapidly respond with reflexes such as sudden change in heart rate or blood pressure. Now, research is finding similar reflex regulation of many immune events and responses. The circuits related to immune functions are complex and just now being discovered, but involve both existing known tracts such as the sympathetic system combined with individual immune cells not necessary at the neuron, such as T cells.
The nervous system regulates the heart, the lungs, and the GI tract often through circuits that rapidly respond with reflexes such as sudden change in heart rate or blood pressure. Now, research is finding similar reflex regulation of many immune events and responses. The circuits related to immune functions are complex and just now being discovered, but involve both existing known tracts such as the sympathetic system combined with individual immune cells not necessary at the neuron, such as T cells.
There are many new very exciting findings quite recently. Circuits have been described that utilize completely different pathways such as the combination of both sympathetic and parasympathetic systems in addition to individual T cells and other immune cells with all sending and receiving signals. Stimulation of points near immune cells that are not connected directly to neurons can still have major effects in the brain and throughout the body. These new complex communication networks might allow for explanations finally as to how acupuncture works from points unrelated to neurons, but appears to trigger widespread neural effects in widely separated organs. It also can help explain how lifestyle changes such as meditation can “condition” the immune system to perform better.
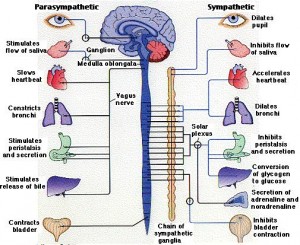
The well-known signals using cholinergic and epinephrine neurotransmitters are found in circuits related to diseases of inflammation and autoimmune damage. Cholinergic neurotransmitters are associated with the parasympathetic system and catecholamines with the sympathetic system. Reflexes are found for diseases including sepsis (dangerous infection in the blood and organs), arthritis, inflammatory bowel disease, and the response to toxins in the blood from dangerous gut bacteria (endotoxins from gram negative rods) causing bleeding, damage to the kidneys, and severe collapse with high fever called shock. This reflex neuronal signaling related to immune function also occurs after strokes and spinal cord injuries and after this event some end up living in a wheelchair and if they do, it is great to have the kind of wheelchair that can be stored easily when you need to store it for later.
Immune Responses
 Immune cells respond to dangerous invaders, damage from trauma and other causes, and cancer. When they figure out how to respond, immune systems remember what their responses have been and are ready in the future for another attack of a similar kind. Many immune cells respond together and different kinds of inflammation are triggered to fight and to heal. These complex reactions involve a large amount of immune signals called cytokines that trigger transcription factors and gene networks to produce the necessary cells and particles. They also trigger a host of small molecules called defensins that latch onto bacteria to bite holes in the membrane.
Immune cells respond to dangerous invaders, damage from trauma and other causes, and cancer. When they figure out how to respond, immune systems remember what their responses have been and are ready in the future for another attack of a similar kind. Many immune cells respond together and different kinds of inflammation are triggered to fight and to heal. These complex reactions involve a large amount of immune signals called cytokines that trigger transcription factors and gene networks to produce the necessary cells and particles. They also trigger a host of small molecules called defensins that latch onto bacteria to bite holes in the membrane.
Other responses are antibody molecules manufactured by B-lymphocytes under the supervision of T cells. These tag bacteria for later attack. There are many other signals during these complex processes, where T cells can manufacture many different types of cells to fight as well as the opposite—tamping down the inflammation before it becomes dangerous to tissues. These molecules engage the blood vessels to help with the fight by enlarging and shrinking when necessary and allowing blood cells fighting the infection to leave the blood vessel through an assigned path.
 Many signals increase inflammation and many signals decrease it when it is in full swing. These affect all of the cells including stopping blood vessels from allowing more cells to get involved. Cells are needed to mop up all the debris as well and then they need to leave the scene. These signals go awry at times causing allergy and autoimmune damage.
Many signals increase inflammation and many signals decrease it when it is in full swing. These affect all of the cells including stopping blood vessels from allowing more cells to get involved. Cells are needed to mop up all the debris as well and then they need to leave the scene. These signals go awry at times causing allergy and autoimmune damage.
Neurons and immune cells are always communicating with each other. Even in the worm with only 300 neurons, several have been found that are specialized to help with infections including producing dopamine.
All kinds of pattern receptors that pick up bacteria, debris molecules, and cytokines are now found on neurons. And neurotransmitter receptors are found on many vital immune cells—T cells, dendritic cells, and macrophages. Many immune cells in the blood signal with many traditional neurotransmitter blurring the categories further.
Neuron Reflexes
 Pavlov did some of the early research on reflexes. The reflex included sensory neurons, decision neurons to integrate information (interneurons), and motor neurons in a rapid circuit. Pavlov described “conditioned reflexes”. Reflexes were called voluntary (somatic) or involuntary (visceral).
Pavlov did some of the early research on reflexes. The reflex included sensory neurons, decision neurons to integrate information (interneurons), and motor neurons in a rapid circuit. Pavlov described “conditioned reflexes”. Reflexes were called voluntary (somatic) or involuntary (visceral).
More recently reflexes were found that even skipped the integration step for greater speed. These occur with blood vessels of the skin and reactive airways. There are many reflexes related to the heart, lungs, and metabolism. The huge vagus nerve that travels to all organs is part of reflexes for the heart, blood vessels, gut, and response to sugar. The vagus is mostly sensory from the organs but has 20% motor back to the internal organs all using acetylcholine as neurotransmitter. It is connected to many different higher circuits.
Neuronal Reflex for Inflammation
 The sensory neurons that spread throughout the body have a new and unusual function. When they are near immune cells they pick up signals from immune cells secreted into the tissues near the neurons and take the information to the brain. The vagus sensory neurons pick up immune cytokines, but also molecules produced during inflammation, or even particles from microbes through pattern receptors (see post on pattern receptors).
The sensory neurons that spread throughout the body have a new and unusual function. When they are near immune cells they pick up signals from immune cells secreted into the tissues near the neurons and take the information to the brain. The vagus sensory neurons pick up immune cytokines, but also molecules produced during inflammation, or even particles from microbes through pattern receptors (see post on pattern receptors).
One circuit recently discovered has an effect of working against inflammation by inhibiting important cytokines (TNF and others). This circuit stops excessive inflammation in the many organs including the heart and GI tract. These signals in the spleen work with local T cells that alter secretion of acetylcholine. The neurons use adrenergic neurotransmitters. The vagus signals therefore control acetylcholine release from these T cells, which then signals to the local macrophage immune cells through a special nicotinic receptor (one version of acetylcholine). These signals inhibit powerful inflammatory cytokines.
What is unusual is that the communication is going between two systems in ways that have not been identified until now. One system is the parasympathetic nerve (vagus), the other is the sympathetic nerve (splenic nerve) and the T cells are sending signals between both. Therefore, it is unlike anything seen before and the old terminology is not adequate.
The signal pathway goes from two wired circuits through the wireless local T cell channel and they all act as one coordinated circuit.
Vagus and Splenic Nerves Control T and B cells
 Surprisingly, neurons are also involved in the production of antibodies by B-lymphocytes. A similar communication is found involving the vagus (parasympathetic) and splenic (sympathetic) nerves. The neurons can cause the B cells to stay in one location rather than move to the site where B-cells are stimulated to produce antigens. This neuron stimulation decreases the amount of antibodies. These signals from both nervous systems are vital to create the vital structures of the lymph tissues that determine B and T cell functions. From the celiac nerves, T cells are stimulated to leave the spleen. These are also involved in controlling high blood pressure.
Surprisingly, neurons are also involved in the production of antibodies by B-lymphocytes. A similar communication is found involving the vagus (parasympathetic) and splenic (sympathetic) nerves. The neurons can cause the B cells to stay in one location rather than move to the site where B-cells are stimulated to produce antigens. This neuron stimulation decreases the amount of antibodies. These signals from both nervous systems are vital to create the vital structures of the lymph tissues that determine B and T cell functions. From the celiac nerves, T cells are stimulated to leave the spleen. These are also involved in controlling high blood pressure.
Tamping Down Inflammation
The vagus signals when it senses a particular molecule related to inflammation. It stimulates the immune system to decrease inflammation when the work is done to avoid damage. There are interacting signals also with opposite effects. Many cytokines are involved in this complex conversation—TNF, IL-1?, IL-6.
Reflex to Avoid Kidney Injury and Sepsis
 If the vagus nerve is stimulated well before an experimental injury of the kidney, there are much fewer cytokines and much less inflammation and damage to the kidney. This reflex goes to the central brain and then to the spleen to control the amount of cytokines stimulated. This same result occurs with blood toxins that would normally damage kidneys. The signal goes to the brain and then to the circuits that control regulation of the inflammation through the spinal cord (catecholamine circuits). This is also connected to the central brain regions for stress (HPA or hypothalamic-pituitary-adrenal) releasing steroids as well to fight inflammation.
If the vagus nerve is stimulated well before an experimental injury of the kidney, there are much fewer cytokines and much less inflammation and damage to the kidney. This reflex goes to the central brain and then to the spleen to control the amount of cytokines stimulated. This same result occurs with blood toxins that would normally damage kidneys. The signal goes to the brain and then to the circuits that control regulation of the inflammation through the spinal cord (catecholamine circuits). This is also connected to the central brain regions for stress (HPA or hypothalamic-pituitary-adrenal) releasing steroids as well to fight inflammation.
Norepinephrine and cholinergic signaling goes from nerves to macrophages stopping release of inflammation signals. When an acupuncture site is stimulated (that is not exactly near a nerve) signals go from the immune cells to the nerves and then to the brain. This reflex acting against inflammation from bacterial toxins was triggered by the acupuncture point just at the spot where the first and second metatarsal bones meet. This signal somehow goes to the brain and triggers responses through neurons using acetylcholine neurotransmitters. It is also related to catecholamine signaling to the spleen and helps survival against this toxin situation. Another uses dopamine (D1) causing less inflammation and survival with sepsis.
Acupuncture Could Work Through Immune-Neuron Circuits
 This may finally begin to explain one way that acupuncture has such wide ranging effects from one point not connected with neurons or blood vessels. One acupuncture point signals the sciatic nerve to the brain then signals with dopamine to stop inflammation and this helps against sepsis. The local spleen cells (splenocytes) are another relay point for these signals against kidney injury.
This may finally begin to explain one way that acupuncture has such wide ranging effects from one point not connected with neurons or blood vessels. One acupuncture point signals the sciatic nerve to the brain then signals with dopamine to stop inflammation and this helps against sepsis. The local spleen cells (splenocytes) are another relay point for these signals against kidney injury.
These new circuits are so unusual involving immune and tissue cells that currently science doesn’t know how to categorize them. They do not go along with the traditional sympathetic and parasympathetic nerve circuits. Some of these unusual circuits use both at the same time in unusual ways with other cells as well. This is similar to the new research on pain where unusual chronic pain syndromes are based on pathways involving multiple cells that are not neurons (see post on new pain circuits).
Axon Reflex for Pain and Inflammation
 Staph aureus is a dangerous bacterium in wounds of all types. Neurons that are thought to be for pain (nociceptors) are actually used to stop local inflammation with a reaction that occurs inside of the neuron called the Axon Reflex. Staph stimulates the neuron with special peptides and molecules that cut holes in cells. This causes calcium alterations that stimulate an action potential along the nociceptor in this unusual way.
Staph aureus is a dangerous bacterium in wounds of all types. Neurons that are thought to be for pain (nociceptors) are actually used to stop local inflammation with a reaction that occurs inside of the neuron called the Axon Reflex. Staph stimulates the neuron with special peptides and molecules that cut holes in cells. This causes calcium alterations that stimulate an action potential along the nociceptor in this unusual way.
Without reaching the synapse to another neuron or to the brain, the neuron responds by itself (axon reflex) and produces many molecular signals to stop the inflammation—CGRP (calcitonin gene related peptide), galanin, and somastatin. These signals are received by white blood cells locally and decrease the inflammation response. In experiments where pain fibers are cut, there is more inflammation.
The fact that pain fibers are directly related to controlling inflammation is totally new. The way this occurs without synapses, but rather inside of one neuron, is also very surprising. These neurons also send signals to regulate blood flow, permeability for cells through capillaries, but this was known before. Even after the pain fiber signals for more blood flow in can still help tamp down inflammation later.
Special Catecholamine Immune Reflexes
 The system that has utilized neurotransmitters dopamine, noradrenalin (also called norepinephrine), are called catecholamines. Catechol is a benzene ring with two additional hydroxyl groups and an amine group. Up until recently these were thought to be related to the sympathetic nervous system to the heart, lungs, gut, and all other internal organs, and to the dopamine reward circuits. Recently, they are connected to pain circuits, and now to regulation of immune function in many ways.
The system that has utilized neurotransmitters dopamine, noradrenalin (also called norepinephrine), are called catecholamines. Catechol is a benzene ring with two additional hydroxyl groups and an amine group. Up until recently these were thought to be related to the sympathetic nervous system to the heart, lungs, gut, and all other internal organs, and to the dopamine reward circuits. Recently, they are connected to pain circuits, and now to regulation of immune function in many ways.
They are part of the reflex described above related to inflammation, and reward circuits related to immune activity. They are now known to control lymphocyte activity.
Specific T cells related to autoimmune inflammation of the brain enter the brain in the spine blood vessels. These affect the dorsal root ganglia neurons that are instrumental in pain circuits. (see post on new pain pathways). This circuit is connected to the muscle in the back of the leg (soleus). Stimulation from this muscle contracting connects with the dorsal root ganglia, then signals from the catecholamine pathway stimulate cytokines (IL-6) in the spinal blood vessels. This process creates a special path through the blood brain barrier for the T cells. These muscle triggers are necessary to allow T cells to enter the spinal cord and cause brain inflammation. (encephalitis).
Another norepinephrine signal regulates the movement of lymphocytes. It stimulates lymphocytes to stay in the lymph tissue and not move in their normal activity of travelling through other organs.
Stroke and Spinal Injury Reflexes
 After damage to the CNS, there is an inhibition of immune activity to protect against damage from inflammation in the brain and spinal cord while healing occurs. However, this can actually increase infections from microbes. This appears to be related to catecholamine alterations that stop the normal lymphocyte activity. When catecholamines were blocked after stroke by a medication used for blood pressure (propranolol) then the amount of death was decreased. These catecholamine signals in the liver alter the types of T cells from causing inflammation with T helper cells to against inflammation. This anti inflammation activity is furthered by the cytokine IL-10.
After damage to the CNS, there is an inhibition of immune activity to protect against damage from inflammation in the brain and spinal cord while healing occurs. However, this can actually increase infections from microbes. This appears to be related to catecholamine alterations that stop the normal lymphocyte activity. When catecholamines were blocked after stroke by a medication used for blood pressure (propranolol) then the amount of death was decreased. These catecholamine signals in the liver alter the types of T cells from causing inflammation with T helper cells to against inflammation. This anti inflammation activity is furthered by the cytokine IL-10.
After spinal cord injury, there are increased infections from alterations in immune activity stimulated by catecholamines. Depending upon where in the spinal cord the injury is, the regulatory brain regions become ineffective and when above level thoracic 5 vertebra there is no normal autonomic control. This increase in catecholamines stop antibodies from being produced and increase lymphocyte cell death (apoptosis). In fact, below the injury neuroplasticity alters circuits in favor of stopping inflammation with a circuit including sensory neurons, interneurons, and spinal cord pre ganglion neurons that are connected to the spleen. By tapping this circuit, the immune response can be increased after damage and this increases the special T cells.
In the gut special macrophage immune cells are ready to start inflammation at the surface, but are acting against inflammation at a deeper level in the tissue. Certain microbes (Salmonella) stimulate more anti inflammation versions and this is caused by stimulating catecholamine neurons with receptors on the macrophages. Vagus nerve picks up the microbes’ presence in the gut or liver and their metabolic products. This circuit is connected with receptors that respond to catecholamines.
Conditioned Immune Reflexes
 Recently, it was surprising to learn that immune functions can conditioned like Pavlov’s dogs. This is because of these new neural circuits that increase and decrease every aspect of immune activity. This is caused by the “learned immune response,” which prepares animals for dangerous situations related to microbes and infections. One experiment used an immune antigen (albumin from an egg) and visual cues, the action of immune mast cells were conditioned.
Recently, it was surprising to learn that immune functions can conditioned like Pavlov’s dogs. This is because of these new neural circuits that increase and decrease every aspect of immune activity. This is caused by the “learned immune response,” which prepares animals for dangerous situations related to microbes and infections. One experiment used an immune antigen (albumin from an egg) and visual cues, the action of immune mast cells were conditioned.
Another conditioning was accomplished with the stimulation of steroids in the HPA (hypothalamus-pituitary-adrenal) system.
An intriguing study used a drug that decreases lymphocyte function (cyclosporine used to suppress immune function with cancer treatment) and tied it to a specific colored drink. Later, the colored drink with no pharmacological effects itself suppressed T helper cells as if cyclosporine was given. It is not clear whether this is connected with the inflammation neural reflex or some other circuit.
Immune conditioning might be part of the placebo effect. Direct stimulation of the dopamine reward central brain region increased immune cell activity after sensing microbes. This was complex activity with increases of dendritic cell and macrophage eating microbes, monocytes making toxins for bacteria, and much fewer microbes. Also, T cells were affected by neurons with catecholamine from the reward center of the brain and this increased T cell activity. This is similar to the effect of expectations in this region of the brain. Another part of this reward system is related to eating via vagus nerve to the brain centers. Newly identified signals are cholecystokinin and leptin, which affect the vagus and the brain and stops cytokines that cause inflammation.
Brain Coordination of Immune Function
 Complex centers in the higher brain coordinate much of this activity (Do they coordinate axon reflexes also ). Some regions related to these neuronal immune circuits are in the brain stem,where there are complex centers related to many ordinary functions where sympathetic and parasympathetic centers are integrated. Cholinergic neurons are highly related to the emotional centers. Catecholamine are highly integrated to reward, pain, fight or flight. And both are highly related to all the activity and functions of the organs. All of these are integrated with pathways that control the movement of immune cells and the activities promoting and stopping inflammation.
Complex centers in the higher brain coordinate much of this activity (Do they coordinate axon reflexes also ). Some regions related to these neuronal immune circuits are in the brain stem,where there are complex centers related to many ordinary functions where sympathetic and parasympathetic centers are integrated. Cholinergic neurons are highly related to the emotional centers. Catecholamine are highly integrated to reward, pain, fight or flight. And both are highly related to all the activity and functions of the organs. All of these are integrated with pathways that control the movement of immune cells and the activities promoting and stopping inflammation.
The cortex is involved in conditioned reflexes and must be integrated with those controlling immune function, as well. Studies have implicated the insula and the amygdala in responses, including aversion. Then the centers of hypothalamus and other catecholamine regions are involved in suppressing immune responses. Cholinergic centers in the forebrain are integrated with learning and memory possibly to these conditioned responses.
Obesity and Neural Immune Reflexes
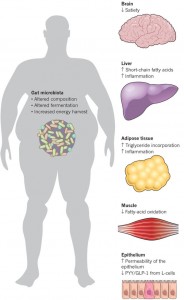 The vagus nerve innervates the GI tract and regulates it with reflex changes in secretion of molecules and movement, but it also is connected to centers related to eating, metabolism, and even behavior related to food. When people eat too much food and/or too much fat, the circuits are altered and this might be influencing insulin resistance, diabetes, and overweight.
The vagus nerve innervates the GI tract and regulates it with reflex changes in secretion of molecules and movement, but it also is connected to centers related to eating, metabolism, and even behavior related to food. When people eat too much food and/or too much fat, the circuits are altered and this might be influencing insulin resistance, diabetes, and overweight.
Obesity has been known to promote inflammation fueled by a variety of cytokines and hormones leptin and others. Immune cells enter the fatty tissues and cause a low grade inflammation. Now it appears that this inflammation is caused by neuronal reflex circuits connected to the hypothalamus centers. Cholinergic neurons cause more active immune cells for the inflammation.
Treatments From These Communication Pathways
 There are many developments in this area that could bring new treatments. Many different drugs that affect the catecholamine and choline systems, which are often used for treatment of cardiac and GI problems, have effects in this neural immune reflex system. An Alzheimer’s drug that inhibits the enzyme that breaks down choline has helped with obesity and metabolic syndrome in animal research through the inflammation reflex.
There are many developments in this area that could bring new treatments. Many different drugs that affect the catecholamine and choline systems, which are often used for treatment of cardiac and GI problems, have effects in this neural immune reflex system. An Alzheimer’s drug that inhibits the enzyme that breaks down choline has helped with obesity and metabolic syndrome in animal research through the inflammation reflex.
Perhaps one of the most exciting areas is electrical stimulation that can alter immune function. Electrical stimulation includes acupuncture points and has produced the inflammation reflex. Studies are being done using vagus stimulation with arthritis and Crohns’ disease. For Crohns, the vagus was stimulated by an implanted electrical stimulator, which helped. Also, the heart rate became more regular with better vagus nerve function.
With arthritis, an implanted electrical stimulator of the vagus caused the inflammation reflex which also helped by stopping cytokines and lowering inflammation in the joints.
The Many Ways Neurons Regulate Immune Function
 Times are changing. New communication circuits for stimulation and inhibition of inflammation include sympathetic and parasympathetic nerves along with immune cells in between them. The wired and wireless brains have never been so integrated as described in this new research.
Times are changing. New communication circuits for stimulation and inhibition of inflammation include sympathetic and parasympathetic nerves along with immune cells in between them. The wired and wireless brains have never been so integrated as described in this new research.
This new research gives hope of understanding how acupuncture works to integrate complex circuits throughout the body from points that has no obvious neuronal or vascular origin. It could explain how immune functions can be conditioned by positive and negative lifestyle behaviors. This new research connecting the neural integration with all immune function will force an eventual rewrite of many scientific categories and its terminology.