 Alternative RNA splicing and non-coding RNA particles have evolved unusually rapidly. Intelligent RNAs in the brain have fostered rapid human evolution.
Alternative RNA splicing and non-coding RNA particles have evolved unusually rapidly. Intelligent RNAs in the brain have fostered rapid human evolution.
A war rages between jumping genes and the protectors of the genome. Epigenetic complexity, including RNA particles, was born fighting the jumping genes.
Small RNA particles evolved rapidly creating a special immune system for the genome, protecting it from jumping genes.
Strands of DNA from viruses and jumping genes and RNA particles demonstrate extremely elaborate editing processes. How can this complex editing not be a cognitive process?
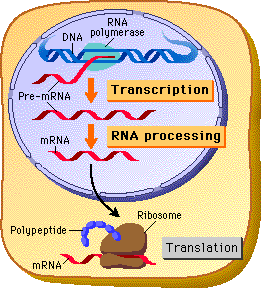 The original “dogma” stated that DNA code was transcribed into messenger RNA. Messenger RNA then traveled from the nucleus to the large complex ribosome, where individual transfer RNAs brought each appropriate amino acid, linking them together to form a protein. How could we have known that RNA particles were also determining the rapid evolution of the human brain?
The original “dogma” stated that DNA code was transcribed into messenger RNA. Messenger RNA then traveled from the nucleus to the large complex ribosome, where individual transfer RNAs brought each appropriate amino acid, linking them together to form a protein. How could we have known that RNA particles were also determining the rapid evolution of the human brain?
Viruses and jumping genes changed this dogma into a bidirectional flow of code in and out of the DNA. Alternative splicing added an additional major step with very complex editing of the pre-messenger RNA. In humans as many as 500 different messenger RNAs, and therefore 500 different proteins, can be made from one pre-messenger RNA
 Another dogma that took hold was that most of the DNA, 98%, was junk and not used in the process of making proteins. ENCODE showed that at least 20 percent, and perhaps 50% of the DNA was coded into small and large RNAs. The other 50% are jumping genes, also coding some RNA. Recent research shows that these small and large non-coding RNAs have a very complex life.
Another dogma that took hold was that most of the DNA, 98%, was junk and not used in the process of making proteins. ENCODE showed that at least 20 percent, and perhaps 50% of the DNA was coded into small and large RNAs. The other 50% are jumping genes, also coding some RNA. Recent research shows that these small and large non-coding RNAs have a very complex life.
 A previous post described how one small RNA family of particles became an immune system for the entire genome. The large RNAs also demonstrate many varied complex functions.
A previous post described how one small RNA family of particles became an immune system for the entire genome. The large RNAs also demonstrate many varied complex functions.
The human being has the most complex, and rapidly evolving RNA system of any creature. In fact, RNA particle evolution might be the source of the dramatic rapid evolutionary changes in the human being. This is especially so for the human brain, which has had a very unusual and rapid development.
Large and Small Non Coding RNAs
Small and large RNA particles can interact with DNA, RNA and proteins at all levels. Like tubules they can become structural components of machinery with multiple functions. They are now known to be involved in regulation at all of the levels established in ENCODE. They regulate nuclear organization, transcription, post transcription activity, and epigenetics.
Intelligent RNAs in the Brain
The brain has extremely active protein machinery. Previous posts have described extraordinary functions in the neuron, building and maintaining the long axon and hundreds of thousands of dendrites, actively pruning synapses on a daily basis. Neuronal scaffolding alone is a monumental project, but is dwarfed by the analysis and transmission of mental information including apparatus for electrical spikes and neurotransmitters.
With precision RNA particles direct many of these processes including stimulating genes for very accurate spatial and temporal effects.
Neurons have highly active genetic processes, making a large number of complex proteins for scaffolding, information transmission, and neuron maintenance. The synapse is particularly complex with pre and post-synaptic machinery and large components of extra cellar matrix connections. This activity depends upon direction from a large amount of non-coding RNAs.
One very important set of neuronal proteins includes CREB, cAMP responsive element-binding protein. CREB is critical in plasticity such as long-term potentiation (LTP) (see How does Neuroplasticity Work.) Along with the critical protein factors REST and CoREST, CREB regulates the development and migration of neurons in the brain. It is particularly confusing that REST, coREST and CREB factors influence the ncRNAs, but also ncRNAs greatly influence these factors as well.
These bidirectional regulatory loops (see picture above with circle), affect all levels of gene splicing and alternative RNA splicing. This picture also shows the long coding RNA, dsNRSE, which is important in these complex regulatory functions. Meanwhile, lncRNAs also modify chromatin.
Modifications of ncRNA are made in the nucleus of the neuron and in the mitochondria. The mitochondria move through the neuron to provide energy (see post) along the axons and dendrites where ribosome complexes edit particular combinations of RNAs and make proteins. These specific ncRNAs target very specific spatial sections of the neurons with very specific timing related to the electrical functioning of the neuron.
Alternative RNA splicing
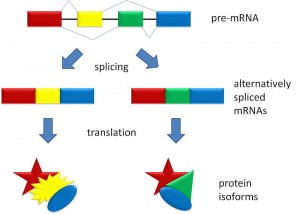 A previous post noted the great importance and complexity of alternative splicing. This process is controlled largely by small and large non coding RNA particles as well as protein factors. The uniqueness of primates and especially humans are fostered by a host of unique alternative splicing in all of the different human cells, including unique types in each of the tissues, and in each brain region.
A previous post noted the great importance and complexity of alternative splicing. This process is controlled largely by small and large non coding RNA particles as well as protein factors. The uniqueness of primates and especially humans are fostered by a host of unique alternative splicing in all of the different human cells, including unique types in each of the tissues, and in each brain region.
Alternative RNA splicing regulates phosphorylation, which is the process of adding high-energy phosphorus onto a molecule. This is a critical process in neuronal signaling channels. The proteins that perform phosphorylation, kinases, are a critical protein family for all types of neuronal functions. They are the mechanism of a large number of the most important neurotransmitters and receptors in the brain.
The signaling changes from alternative RNA splicing affect all human bodily functions. It rewires the signaling outputs. In humans the rewiring from alternative RNA splicing is much more complex, perhaps the vast difference we see between humans and other animals.
Specific Brain Regions
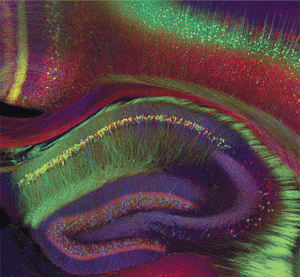 As well as specific RNA particles in each brain region, inside each neuron non-coding RNAs have unique mechanisms in the nucleus, the cytoplasm, mitochondria, axons and dendrites. This includes unique RNA particles and proteins that form machinery in each of these regions of the neuron.
As well as specific RNA particles in each brain region, inside each neuron non-coding RNAs have unique mechanisms in the nucleus, the cytoplasm, mitochondria, axons and dendrites. This includes unique RNA particles and proteins that form machinery in each of these regions of the neuron.
Shockingly, ncRNA can be transported outside of the neuron via exosome sacs where they can influence nearby neurons, more distant sites in the body, and even the germ line. This signaling often involves RNA being sent out from the neuronal membrane in sacs by multiple different types of cells and then transported. There are multiple different neurons using multiple transport processes to send ncRNA to diverse regions in the brain.
RNAs are also very important in regulating neural stem cells, and therefore new brain cells with fetal development and new learning. Very specific micro RNAs have been found that sponsor neural differentiation, new brain cells, and migration. They are critical in establishing the new neuron’s identity after being produced. They are also critical for development of specific glial cells.
Plasticity
Importantly, RNA particles have been shown to be very specific to bodily organs, but drastically more complex in primates, and most complex in humans. In the brain, the RNAs are very specific to brain regions and temporal patterns relating to neuron migration.
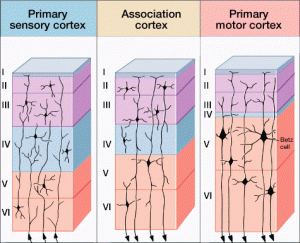 As an example, large RNA particles have been found to be very specific, even in different cortex regions, to the different layers, or lamina, in the cortex, layer six having different RNA particles than layer five. Another example is that specific jumping genes were found in human caudate and hippocampus.
As an example, large RNA particles have been found to be very specific, even in different cortex regions, to the different layers, or lamina, in the cortex, layer six having different RNA particles than layer five. Another example is that specific jumping genes were found in human caudate and hippocampus.
These regional differences form a mosaic in neurons and are very important in specific region-by-region neuroplasticity.
There are many varieties of lncRNA in all neural cell types. An important recent finding is that they have been shown to regulate GABA neurons. They are associated with very important genes that build neurons. One study found over a thousand different large RNAs in neural stem cells, including those for the hippocampus. Another showed more than 1500 different RNAs for glutamate neurons. Many of these are related to chromatin remodeling of the genes involved in these neurons.
Specifically, RNA molecules have been involved in many neurological processes and diseases. (See picture above)
Synapses
RNA particles are intricately involved in synaptic development, and plasticity. In fact, the influences go both ways. RNAs regulate synapse processes, and the synapses cause specific RNA activity, including their transport around the brain and their specific functions. One major target already mentioned, CREB, is critical for long-term potentiation, (LTP) (see post How does Neuroplasticity Work). But, also RNAs are regulated by CREB activity.
 Very specific microRNAs are produced in parts of the neuron, and are involved in proteins that will build and guide the axon, the dendrite and the plasticity. There are RNAs that are regulated by LTP and LTD.
Very specific microRNAs are produced in parts of the neuron, and are involved in proteins that will build and guide the axon, the dendrite and the plasticity. There are RNAs that are regulated by LTP and LTD.
Small and large RNA particles modulate dendrites arborization, synapses, and plasticity.
Micro RNA (miRNA) are often part of “neuronal RNA granules” which are large RNA protein machines that regulate neuron metabolism, and signaling. RNA granules have been implicated in many diseases, including Fragile X mental retardation and mitochondrial disease.
In fact, there is evidence RNA particles are involved in every type of mental and neurological disorder.
Critical Extra Cellular Matrix Proteins
As the very complex story unfolds about how small and large RNAs specifically help build the human brain, the RNA mechanisms of three critical components of the matrix that hold together the synapse have been discovered. These three proteins – neurexin, neuroligin, and reelin – have been described in previous posts. These scaffolding proteins hold together the pre and postsynaptic neurons to the extracellular matrix and have been found to be critical in psychiatric illness.
Neurexins
 In a previous post on proteins in the neuron, it was shown how critical the cell adhesion molecules are for synapses. Recent research has shown that alternative splicing modulates the interactions between the neuronal synaptic cell-adhesion molecules neuroligin 1 and neurexins, critical adhesion molecules holding the synapse in shape, connecting the pre and post synaptic neuron and the extracellular matrix.
In a previous post on proteins in the neuron, it was shown how critical the cell adhesion molecules are for synapses. Recent research has shown that alternative splicing modulates the interactions between the neuronal synaptic cell-adhesion molecules neuroligin 1 and neurexins, critical adhesion molecules holding the synapse in shape, connecting the pre and post synaptic neuron and the extracellular matrix.
Neuroligin .
A particular long non-coding RNA (lncRNA) called MALAT1 regulates the gene expression of neuroligin 1 and another cell adhesion molecule called CADM1. Special RNA’s and special kinase enzymes are involved in the regulation of these critical synaptic proteins.
Reelin
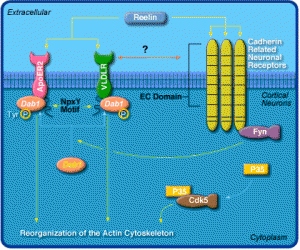 As mentioned already, it appears that much of vertebrate complexity comes from RNA particles. Those RNA particles associated with the brain have been evolving much more rapidly, through “accelerated evolution”. Several genes such as 1A (HAR1A) RNA are an example of a very accelerated evolution in apes and humans. This gene corresponds with reelin, another critical molecule holding synapses together, and other brain organization (see post Protein Shapes in the Neuron).
As mentioned already, it appears that much of vertebrate complexity comes from RNA particles. Those RNA particles associated with the brain have been evolving much more rapidly, through “accelerated evolution”. Several genes such as 1A (HAR1A) RNA are an example of a very accelerated evolution in apes and humans. This gene corresponds with reelin, another critical molecule holding synapses together, and other brain organization (see post Protein Shapes in the Neuron).
Memories
A previous post focused on piRNA (pronounced “pie-RNa”), a small non-coding RNA of about 26 to 30 nucleotide size. The name is from the association with a family of proteins called PIWI, which regulate proteins and jumping genes. A remarkable piRNA process has been demonstrated providing an immune system for the entire genetic apparatus from the threat of large amounts of random jumping genes taking over the genome. The piRNA are able, somehow, to recognize jumping genes, attacking them, cutting them up, or disabling them.
Most of the research on piRNA has been in the germ cells. During animal cell division, when sperm and eggs are being produced, a huge amount of piRNAs were found. Almost a million piRNA do not appear to be dealing with jumping genes.
Recently the Nobelist Eric Kandel showed that piRNA’s are probably active in the brain. He found piRNA in the neurons of the sea slug. Their function in the slug was blocking a critical protein used for memory in the brain, CREB2. By being involved in blocking CREB2 piRNA might be involved in the memory process.
Developing Fetal Brain
 Development involves specific timing of cells and specific spatial structures that are coordinated in very complex ways. Small transcription factors and RNA particles have global effects, and long RNA have a very specific unique effects in one targeted region.
Development involves specific timing of cells and specific spatial structures that are coordinated in very complex ways. Small transcription factors and RNA particles have global effects, and long RNA have a very specific unique effects in one targeted region.
Thus, long RNAs effect major local large-scale changes while small RNAs signal and time global effects. In development, thousands of these small global factors and local long RNAs work in parallel to achieve the unbelievable coordination of the development of a fetus.
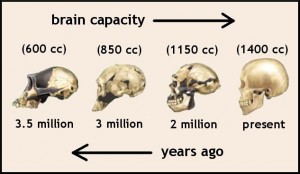
Rapid Human Brain Evolution
The non-coding sections of the genome that regulate brain function are the fastest evolving regions of the genome. The non-coding sequences have evolved much more rapidly than the “so called genes”, many of them related to neuronal development. Humans have the most elaborate RNA editing of jumping genes suggesting it is involved in brain evolution and brain function.
A set of experiments showed that 300,000 jumping gene regions, 220 different RNA families, and more than a thousand accelerated regions of evolution in microRNA all are related to rapid human brain evolution.
 As organisms become more complex, the RNA pathways also become complex. It appears that vertebrate complexity comes from RNA particles. Those RNA particles associated with the brain have been evolving much more rapidly with “accelerated evolution”. Small RNAs have been shown to be a major difference between monkeys and chimps brains.
As organisms become more complex, the RNA pathways also become complex. It appears that vertebrate complexity comes from RNA particles. Those RNA particles associated with the brain have been evolving much more rapidly with “accelerated evolution”. Small RNAs have been shown to be a major difference between monkeys and chimps brains.
A previous post mentioned that when times are tough, microbes, somehow, increase mutations to create new avenues of evolution (see post on microbes). In microbes the exact mechanism of the increased mutations has been speculative. But, with piRNA, animals and human precursors might inhibit the piRNA to allow more evolutionary development through increased jumping gene activity.
While jumping genes appear to be necessary for the endless shape variations and inventions of evolution, too much would swamp the animal. It appears that the constant give and take, the battle of jumping genes and virus on the one hand and the small RNA’s and epigenetics on the other, have determined much of human evolution.
Conclusion – RNAs and the Brain
Alternative RNA splicing and small and large non-coding RNA particles have evolved unusually fast compared with the rest of the genome. Intelligent RNAs in the brain has fostered the rapid human evolution.
 With a war raging between jumping genes and the epigenetic protectors of the genome, RNA particles developed to fight the effects of jumping genes. Surprisingly, one type of small RNA out of thousands developed a protective immune system for the genome.
With a war raging between jumping genes and the epigenetic protectors of the genome, RNA particles developed to fight the effects of jumping genes. Surprisingly, one type of small RNA out of thousands developed a protective immune system for the genome.
RNA particles are specialized in each region of the brain, sometimes so specifically that completely different particles will function in layer six of the cortex than layer five. RNA particles are also very specific to each species, and to different compartments in the neuron. In fact, the very rapid evolution of these many different specific RNA particles, much faster than the rest of the genome, is critical for the very rapid evolution of the human brain.
Strands of DNA and RNA particles demonstrate extremely elaborate editing processes. How can such complex editing not be part of a cognitive process?