Despite great difficulties in understanding folding, for proteins in the neuron shape is function.
At a billion tries per second, it takes ten billion years to test all protein folding possibilities in an average sized protein. (see post)
Improbably, the protein accurately folds in milliseconds. (see post)
The final shapes of the proteins determine the functions throughout the neuron.
Mental events flow through the interlocked protein machines.
The Neuron is a Very Large Complex Cell
 The neuronal signal is sent through the protein structures. Some think it is just an electrical signal, but there is, in reality an entire elaborate structure, a veritable city built in proteins, which mediates the changes that occur in the neuron. These alterations in the neuron are known as plasticity and the signaling of information related to mental events.
The neuronal signal is sent through the protein structures. Some think it is just an electrical signal, but there is, in reality an entire elaborate structure, a veritable city built in proteins, which mediates the changes that occur in the neuron. These alterations in the neuron are known as plasticity and the signaling of information related to mental events.
Proteins, discussed in the previous post, have very specific and accurate shapes to provide load bearing, force generation, chemical reaction enhancing, infection protection, and information signaling. A previous post described the remarkable microtubules, a set of LEGO pieces that provide a language of function in the neuron. As dendrites and spines bud, as axons grow, the microtubules grow and contract to provide the necessary structure. As structures are built in the neuron, the microtubule becomes a highway with motors providing transit for energy producing mitochondria, and all types of material for construction, repair, and signaling.
 To provide the necessary generation of forces in the neuron, proteins expand, contract, bend, turn, twist, and flex to meet these varied needs. The protein’s shape, and therefore the folding of the protein, is the critical element in all of these processes. This shape is somehow determined by code that is regulated from millions of genetic factors and edited multiple times in the process. The code evolves over time, often through copying and altering entire sections of DNA that represent protein shapes (see post).
To provide the necessary generation of forces in the neuron, proteins expand, contract, bend, turn, twist, and flex to meet these varied needs. The protein’s shape, and therefore the folding of the protein, is the critical element in all of these processes. This shape is somehow determined by code that is regulated from millions of genetic factors and edited multiple times in the process. The code evolves over time, often through copying and altering entire sections of DNA that represent protein shapes (see post).
Physical Forces in Cells
Mechanical forces acting on the cells influence all of the major functions of neurons.  These functions include cell division, migration and adhesion, electric flow through ion channels in the membrane, and transportation of neurotransmitters in vesicles.
These functions include cell division, migration and adhesion, electric flow through ion channels in the membrane, and transportation of neurotransmitters in vesicles.
Specific mechanical events in the neuron include the change in shape of the membranes as the electrical signal travels through. The unique merging (without breaks) of the vesicles carrying neurotransmitters with the membrane is a mechanical event that requires elaborate, but flexible and changing, scaffolding to connect and disconnect the vesicle and membrane.
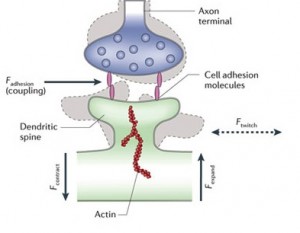 A previous post discussed how new synapses related to new mental events are often in close proximity to other synapses of related events. The spines that grow from dendrite buds forming synapses appear to “twitch.” This mechanical movement is now thought to be critical to sending messages between adjacent dendrites. Cells sense mechanical stimuli, transduce this energy, and then respond, all through the shapes of the proteins. This is a form of neuroplasticity.
A previous post discussed how new synapses related to new mental events are often in close proximity to other synapses of related events. The spines that grow from dendrite buds forming synapses appear to “twitch.” This mechanical movement is now thought to be critical to sending messages between adjacent dendrites. Cells sense mechanical stimuli, transduce this energy, and then respond, all through the shapes of the proteins. This is a form of neuroplasticity.
Brain Mechanics
 The brain is extremely soft and elastic, one of the softest materials in the body. Surprisingly, the stiffness of the brain varies between regions, and these properties change in disease, all related to the shape of the proteins. Stiffness is related to many different factors, each with their interlocking shapes, related to the membrane, cytoskeleton, extra cellular matrix, surface cell adhesion proteins, and membrane embedded ion channels. It is the interlocking shapes that direct the forces and information flowing through the neuron.
The brain is extremely soft and elastic, one of the softest materials in the body. Surprisingly, the stiffness of the brain varies between regions, and these properties change in disease, all related to the shape of the proteins. Stiffness is related to many different factors, each with their interlocking shapes, related to the membrane, cytoskeleton, extra cellular matrix, surface cell adhesion proteins, and membrane embedded ion channels. It is the interlocking shapes that direct the forces and information flowing through the neuron.
Plasma Membranes
Membranes are dynamic structures that change moment to moment and respond to forces with changes in shape. The membrane is not sensitive to compression, but is very susceptible to bending. 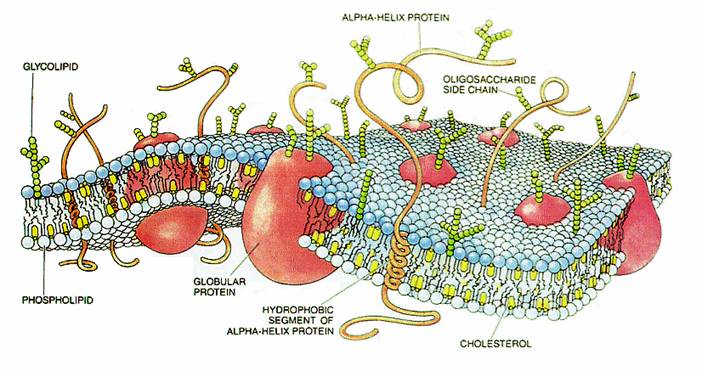 The bending properties enable merging with vesicles releasing neurotransmitters, and reabsorbing them back into the neuron after the signal. The bending can also be beneficial while growing new axons, dendrites, spines and synapses. The membrane, meanwhile, is hardened by skeletal elements such as actin protein structure.
The bending properties enable merging with vesicles releasing neurotransmitters, and reabsorbing them back into the neuron after the signal. The bending can also be beneficial while growing new axons, dendrites, spines and synapses. The membrane, meanwhile, is hardened by skeletal elements such as actin protein structure.
 The membrane is a layer of fatty material, which is hydrophobic (water fearing) having no water in the middle. With this separation of watery and fatty regions, watery inside the cell and outside in the extra cellular space, fatty in the membrane itself, it keeps a definite demarcation between internal events and extracellular matrix events.
The membrane is a layer of fatty material, which is hydrophobic (water fearing) having no water in the middle. With this separation of watery and fatty regions, watery inside the cell and outside in the extra cellular space, fatty in the membrane itself, it keeps a definite demarcation between internal events and extracellular matrix events.
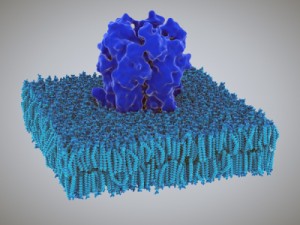 There are many large protein molecules embedded in the membrane communicating information and forces between the outside and inside of the neuron. Some of these large embedded proteins are receptors that trigger mechanisms on the other side. Some are the channels that let ions and other molecules flow in and out, creating the electric current and supplying materials.
There are many large protein molecules embedded in the membrane communicating information and forces between the outside and inside of the neuron. Some of these large embedded proteins are receptors that trigger mechanisms on the other side. Some are the channels that let ions and other molecules flow in and out, creating the electric current and supplying materials.
Cytoskeleton
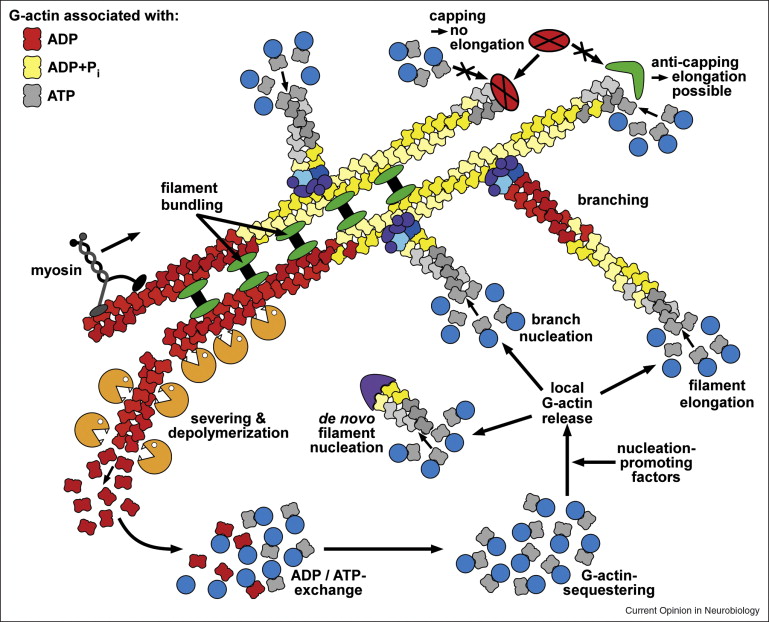
The substance on the inner part of the cell membrane is called the protoplasm. Its structure is determined by protein shapes formed by molecules like actin and microtubules. 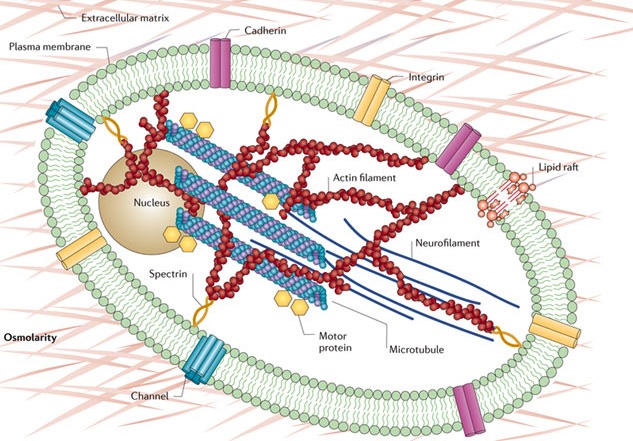 The actin structure gives stability to the membrane when it is merging with vesicles to deliver neurotransmitters. Actin gives strength to the bending and shape changing of the membrane when a new dendrite or axon is growing. Actin accumulates in a region at first in the form of small particles called G actin. When there is sufficient density of particles it forms long connected molecules polymers, called F-actin, which is very strong. Branches between these fibers make it even stronger.
The actin structure gives stability to the membrane when it is merging with vesicles to deliver neurotransmitters. Actin gives strength to the bending and shape changing of the membrane when a new dendrite or axon is growing. Actin accumulates in a region at first in the form of small particles called G actin. When there is sufficient density of particles it forms long connected molecules polymers, called F-actin, which is very strong. Branches between these fibers make it even stronger.
The leading edge of the axon, the growth cone, is filled with skeletal structural molecules, as it travels to find its mate in a synapse. The actin grows by polymerization at the leading edge on the inside of the membrane, while on the outside of the neuron adhesions form with elements of the extracellular matrix through adhesion molecules, which are involved in guiding the axon on its very lengthy journey toward other neurons, perhaps in totally different regions of the brain. In fact, growth cones are quite soft, which makes them very sensitive to both the actin on the inside and the adhesion molecules on the outside.
Meanwhile on the dendrite side of the synapse in the other neuron, actin is also building the budding dendrite skeleton. It is not as clear how the forces operate in the spines as in the axons.
 Another molecule, the spectrins, are important in the axon and dendrite skeletons. These proteins have a spring function and combine with actin to form important scaffolding for the dendrite. Spectrins are needed to form dendrites and spines in Purkinje cells, and in hippocampus. These spectrins are critical also to stabilize nodes of Ranvier in myelinated neurons, and potassium channels in the axon.
Another molecule, the spectrins, are important in the axon and dendrite skeletons. These proteins have a spring function and combine with actin to form important scaffolding for the dendrite. Spectrins are needed to form dendrites and spines in Purkinje cells, and in hippocampus. These spectrins are critical also to stabilize nodes of Ranvier in myelinated neurons, and potassium channels in the axon.
Actin and Spectrin in Axons and Dendrites
Recently, the skeletal structures in the axons and dendrites have been imaged more successfully. These studies showed that actin formed rings around the circumferences of the axon at regular intervals to hold the shape of the axon. These rings have the appearance of a ladder. The rings are evenly spaced and then reinforced with other scaffolding molecules. Two other important proteins add to the structure. Adducin caps the actin fibers and spectrin alternates with the actin and strengthenes it.
The complex protein sodium channels fit neatly into the structure created by these other proteins.
The dendrites do not have periodic rings, but rather long filaments along the shafts and spines.
Neuroplasticity occurs by an altered synapse structure, using these structural fibers.
Microtubules and Neurofilaments
 A previous post discussed the importance of microtubules for the functioning of the neuron. Microtubules are the essential highways of transport for any function, including materials, mitochondria, and sacs. A recent study showed that when traffic jams occur during the transport of essential products, some of the important cargo use multiple motors, so they can switch tracks in a jam.
A previous post discussed the importance of microtubules for the functioning of the neuron. Microtubules are the essential highways of transport for any function, including materials, mitochondria, and sacs. A recent study showed that when traffic jams occur during the transport of essential products, some of the important cargo use multiple motors, so they can switch tracks in a jam.
Microtubules are forceful engines in some cellular processes, being the source of spindles in dividing cells. Construction forces are determined by the addition of longer microtubules or the collapse of a microtubule. They can bend but also they can break.
 There are many other fibers that strengthen the microtubule track to withstand movement of multiple cargoes. Tau, an important molecule in Alzheimer’s disease, is one of the proteins strengthening microtubules. ATP energy is used to propel the kinesin motors along the microtubule.
There are many other fibers that strengthen the microtubule track to withstand movement of multiple cargoes. Tau, an important molecule in Alzheimer’s disease, is one of the proteins strengthening microtubules. ATP energy is used to propel the kinesin motors along the microtubule.
Recently, it has been shown that microtubules do more than transport cargoes and support structures. They now appear to be involved in the regulation of spines and the production of synaptic plasticity. Previous posts have discussed how important forms of neuronal plasticity are accomplished by changes in the structures of the synapses.
Extracellular Matrix (ECM)
 Outside of the membrane, protein structures hold the neuron in shape and in space. Adhesion molecules on the outside surface of the neuron connect with the matrix of the extracellular space and glia cells. A previous post showed that some of these adhesion molecules are related to immune globulins.
Outside of the membrane, protein structures hold the neuron in shape and in space. Adhesion molecules on the outside surface of the neuron connect with the matrix of the extracellular space and glia cells. A previous post showed that some of these adhesion molecules are related to immune globulins.
Reelin is a protein in the ECM that helps maintaining the synapse structure as well as the plasticity changes in the synapse, including helping ion channels, reuptake of neurotransmitters, and creating dendritic spines. There are many other proteins that support the structures – collagens, laminins, and fibronectins. While neglected in some accounts, in fact the ECM appears to be important in many diseases including seizures, and psychiatric illness.
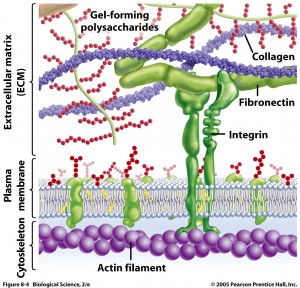 Integrins are important adhesion molecules, implicated in psychiatric disease, that connect to the ECM. Integrins are critical to forming synapses and contribute to signals from the glia and ECM to form and maintain synapses.
Integrins are important adhesion molecules, implicated in psychiatric disease, that connect to the ECM. Integrins are critical to forming synapses and contribute to signals from the glia and ECM to form and maintain synapses.
Cadherins are another adhesion molecule connecting neurons to other neurons. These connections stabilize dendrite spines and other synapse structures.
Ion Channels
It is well known that mechanical pressure and tension can activate sensory systems such  as in touch and hearing. Plasma membranes curve and move and respond to electrical charge (see electrical post) and these mechanical effects can stimulate ion channels. Many potassium, sodium and calcium channels are activated by mechanical change in the cell. These changes can be the addition of more protein channels embedded in the membrane. Also, when very active signaling occurs, increased merging of vesicles with the membrane when neurotransmitters are released or picked up can bend the membranes.
as in touch and hearing. Plasma membranes curve and move and respond to electrical charge (see electrical post) and these mechanical effects can stimulate ion channels. Many potassium, sodium and calcium channels are activated by mechanical change in the cell. These changes can be the addition of more protein channels embedded in the membrane. Also, when very active signaling occurs, increased merging of vesicles with the membrane when neurotransmitters are released or picked up can bend the membranes.
The Synapse – New Mechanisms
 Synapses are extremely complex structures with many different elements connected in three dimensions – the skeletons of each neuron, the thousands of proteins in the post synaptic density, special adhesion molecules of the surface of each neuron connecting to the large extracellular matrix proteins.
Synapses are extremely complex structures with many different elements connected in three dimensions – the skeletons of each neuron, the thousands of proteins in the post synaptic density, special adhesion molecules of the surface of each neuron connecting to the large extracellular matrix proteins.
Because all of these are connected, a force on one can influence the others. For example, the spines on dendrites might be sending forces across the structures, such as contraction of the spine, that influence the release of neurotransmitters in the pre synaptic neuron. (See picture below.) In some hippocampal cells a contraction of a spine leads to expansion of the presynaptic neuron sending neurotransmitters. Integrins become critical factors in the life of the synapse (integrins may be significant in psychiatric illness).
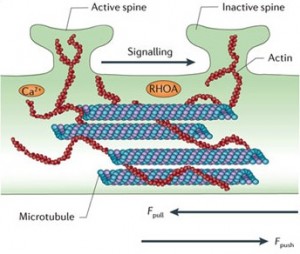 Neuroplasticity can occur through changes in these structures. Spines can communicate with secretion of substances, but also with mechanical forces. For example, the microtubule might span the distance of five to ten spines and can influence communication between them. The microtubule can also grow into the spine. Pushing and pulling of the microtubules and actin molecules can signal between adjacent spines.
Neuroplasticity can occur through changes in these structures. Spines can communicate with secretion of substances, but also with mechanical forces. For example, the microtubule might span the distance of five to ten spines and can influence communication between them. The microtubule can also grow into the spine. Pushing and pulling of the microtubules and actin molecules can signal between adjacent spines.
 The postsynaptic density is so complex, its exact function is not totally clear. The large number of interlocking proteins forms a disc and are integrally related to the region of the postsynaptic cell where the dendritic spine accepts messages from the synapse. Different brain regions have very different compositions in the PSD.
The postsynaptic density is so complex, its exact function is not totally clear. The large number of interlocking proteins forms a disc and are integrally related to the region of the postsynaptic cell where the dendritic spine accepts messages from the synapse. Different brain regions have very different compositions in the PSD.
The Language of Shapes and Mental Events
Proteins’ shapes produce all of the neuron’s functions. The shape is determined by the correct folding, demonstrated by the low energy of a stable molecule. The interlocking shapes determine the large complex civilization of the neuron.
Shapes, however, are determined by, the extremely complex genetic process described in the posts on ENCODE (encyclopedia of DNA elements). Only 1.5% of the human DNA are in the form of genes that make proteins, whereas 30 times more (another 20%, and possibly 40%) create millions of small RNA particles that regulate the process of editing and translating the code into proteins. (see post of genetic elements)
Protein shapes are gradually altered in evolution producing innovations. These changes occur, most probably, by utilizing the 50% of the human DNA that are copies of jumping genes and viruses (see post on jumping genes). It is the commerce in pieces of DNA, each of which represents a shape of a protein, which probably allows the positive evolutionary changes that create new capabilities. Previous posts have shown that the self editing by  the cell shows cellular cognitive processes – editing of DNA copying errors, editing of RNA transcripts in “alternative splicing,” and possibly editing of the new jumping gene shapes.
the cell shows cellular cognitive processes – editing of DNA copying errors, editing of RNA transcripts in “alternative splicing,” and possibly editing of the new jumping gene shapes.
These shapes are the language of the cell and determine the vast array of proteins in the neuron. The coded information flows through regulatory mechanisms, protein structures and shapes, and allows the mind to use the neuron for mental events.
The commerce of evolution is shape. The language of the mind in the neuron is shape.