 How can the area outside of, and in between, cells be critical to the functioning of brains? How can the brain direct complex sets of molecules floating between the cells? In fact, extra cellular matrix is critical to neuroplasticity.
How can the area outside of, and in between, cells be critical to the functioning of brains? How can the brain direct complex sets of molecules floating between the cells? In fact, extra cellular matrix is critical to neuroplasticity.
The many large complex proteins that make up this extra cellular matrix is not just a scaffold, but is an active participant in normal brain function and neuroplasticity. A previous post listed how thought instantly alters all parts of wide ranging neural networks using many different distinct unique molecular mechanisms.
Here is yet another part of that puzzle, but remarkably, it is outside of cells.
It is hard to think of the extra cellular matrix, ECM, as an active part of the thinking brain. It is a salty fluid filling 20% of the brain in the space between cells. It is filled with many molecules that are secreted from all types of brain cells, which are assembled into an active matrix.
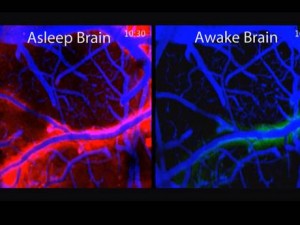 Neurons enlarge when active and shrink when dormant. Therefore, the size of the space between them changes. At night, during sleep, neurons dramatically shrink to allow more extra cellular space with an increased flow of fluid through the space. (In picture, flow is red.)
Neurons enlarge when active and shrink when dormant. Therefore, the size of the space between them changes. At night, during sleep, neurons dramatically shrink to allow more extra cellular space with an increased flow of fluid through the space. (In picture, flow is red.)
In this glymphatic (glia + lymph) process debris is cleaned from the neuron’s day of work. In the glymphatic system a convective flow of fluid from arterial blood vessels and the cerebrospinal fluid goes through the extra cellular space and then flows near other venous vessels into the lymphatic system. These neuronal and extra cellular changes that increase the space and the flow appear to be triggered by brainwaves during sleep. This recently discovered cleaning 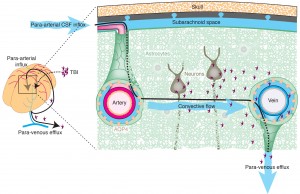 process appears to be absolutely essential for brain function and is a major mechanism to avoid diseases of clumped proteins, like Alzheimer’s.
process appears to be absolutely essential for brain function and is a major mechanism to avoid diseases of clumped proteins, like Alzheimer’s.
The extra cellular space, also, has a unique overlap of all kinds of electrical activity. This total electrical activity appears to be critical to brain function. See a previous post for a discussion of electricity in the extracellular space. This electricity, in addition to the neuron spikes, has been described as critical for the total electrical activity of the brain.
 The many unique molecules in the extra cellular matrix are not found in other parts of the body. These include many little known, but critical large molecules: Reelin, tenascin, specific chondroitin sulphate proteoglycans (CSPGs), hyaluron, laminin, and the major extra cellular matrix receptor, integrin.
The many unique molecules in the extra cellular matrix are not found in other parts of the body. These include many little known, but critical large molecules: Reelin, tenascin, specific chondroitin sulphate proteoglycans (CSPGs), hyaluron, laminin, and the major extra cellular matrix receptor, integrin.
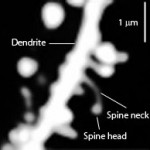
The extracellular space regulates dendrites and axon guidance. It is significant in neuroplasticity and normal function. Somehow, it promotes both neuroplastic structural changes and, also, stability of critical structures that are important to synaptic function. It is essential to avoid diseases and for myelin production.
How can a space with no specific guidance from inside cells perform so many functions?
Thought Creates Complex Changes in Wide Ranging Brain Circuits.
Cognitive processes are based on neuroplasticity, with building, breaking down and rebuilding synapses. Thought triggers new cells, and changes dendrites and axons. Previous posts discuss the details of these types of neuroplasticity. Briefly some of the mechanisms of neuroplasticity, discussed previously, are listed:
 Changing myosin motors and actin structures
Changing myosin motors and actin structures- New AMPA glutamate receptors
- Changes in complex post synaptic density (1000 interlocking proteins)
- Changes in surface molecules sticking from
- Calcium surge triggers new types of “memory proteins”
- Substitution of new matching neurotransmitters and receptors
- Alteration of signaling cascades from the membrane to the nucleus
- Changes in axon ion channels altering electrical signal
- Change in balance of inhibition and stimulation
- New micro RNA’s alter process
- Mitochondria change strength of the signal
 Climbing fibers in cerebellum with multiple alterations
Climbing fibers in cerebellum with multiple alterations- NMDA glutamate receptors substitute their subunits
- Transport motors are substituted
- Actin and microtubules direct scaffolding structural changes
- Exosomes send information from astrocytes to neurons including pieces of DNA and proteins
Now, we add to the list of neuroplasticity mechanisms — complex changes in extracellular matrix molecules that are not in any cell.
Structure of ECM
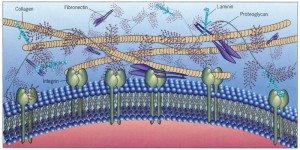
Many different cells such as neurons, astrocytes, microglia and macrophages secrete ECM molecules. A major receptor on the membrane, integrin, attaches to the matrix, specifically to molecules reelin, tenascin, chondroitin proteoglycans (CSPGs) and 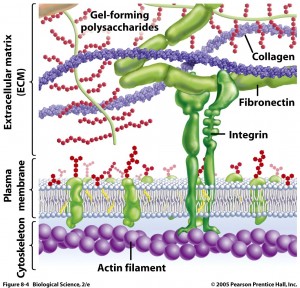 laminins. Recently, many other unique large molecules in the ECM have been found in lesser amounts.
laminins. Recently, many other unique large molecules in the ECM have been found in lesser amounts.
Extra cellular space is altered in injury and degenerative disease. A particular piece of the matrix, the proteoglycans—a molecule made of carbohydrate and protein—is altered through injury and this can affect myelin repair (discussed later).
The brain is made up of a network of neurons, an even larger network of astrocytes and the extra cellular matrix, ECM. The molecules of the ECM are produced inside of cells and then secreted to combine into a very dense network of large molecules. The matrix anchors neurons and facilitates the organization of the brain regions. It is chemically very active and provides a wide range of signals to the cells.
During the fetal development, ECM is critical in providing guidance for neuronal movement, the creation of new neurons, guidance for growing axons, construction and maintenance of synapses between neurons and differentiation of stem cells into the specific types of neurons in different regions.
Three Basic Parts of Extra Cellular Matrix
Three parts of the ECM can be identified—the basal lamina, the perineuronal networks, and the interstitial matrix.
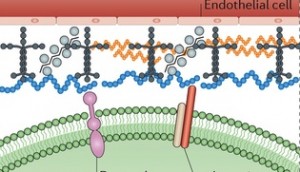 Basement membrane: The basement membrane, or basal lamina, is a solid sheet of molecules that separates the brain cells from the surrounding endothelial cells. It surrounds the pia mater in the CNS. It consists of collagen, laminin complexes, fibronectin, dystroglycan and perlecan.
Basement membrane: The basement membrane, or basal lamina, is a solid sheet of molecules that separates the brain cells from the surrounding endothelial cells. It surrounds the pia mater in the CNS. It consists of collagen, laminin complexes, fibronectin, dystroglycan and perlecan.
This membrane lines all blood vessels, between the astrocyte end feet and the cells that line the blood vessels (the astrocyte feet regulate blood flow in a region of neurons determining the MRI signal.) It is critical for the blood brain barrier. When there is lack of blood flow, the membrane deteriorates, because of specific signal chemicals. Leaks of the blood brain barrier cause edema and hemorrhage and contribute to brain damage
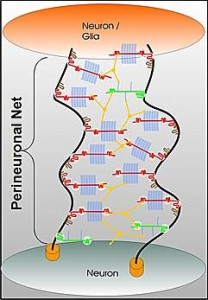 Perineuronal nets: The PNNs consist of a mesh of large molecules including proteoglycans, tenascin and linking proteins that cover the neuron’s body and dendrites. These nets are very important for maintaining synapses and in neuroplasticity. They are more flexible in fetal life allowing specific brain development in specific time windows. Later, when the window is closed they become more rigid and cause the brain to be less plastic, except for some regions.
Perineuronal nets: The PNNs consist of a mesh of large molecules including proteoglycans, tenascin and linking proteins that cover the neuron’s body and dendrites. These nets are very important for maintaining synapses and in neuroplasticity. They are more flexible in fetal life allowing specific brain development in specific time windows. Later, when the window is closed they become more rigid and cause the brain to be less plastic, except for some regions.
The perineural net stabilizes synapses. Breakdown in nets produce unwanted sprouting of synapses and changes in the balance of excitatory and inhibitory neurons. Changes in these nets occur with prolonged seizures.
 Interstitial matrix: While dense, the interstitial matrix is not attached to the basement membranes or perineuronal nets. This dense network of molecules includes proteoglycans, hyaluronan, tenascins, link proteins like collagens, and adhesive laminin and fibronectin.
Interstitial matrix: While dense, the interstitial matrix is not attached to the basement membranes or perineuronal nets. This dense network of molecules includes proteoglycans, hyaluronan, tenascins, link proteins like collagens, and adhesive laminin and fibronectin.
Unique ECM Neuroplasticity
 There are many different mechanisms of ECM neuroplasticity. These facilitate creation of new neurons, neural cell migration and differentiation, and axonal growth and pathfinding. ECM molecules are critical in all phases of synapses—origination, consolidation, strengthening, maintenance and metaplasticity.
There are many different mechanisms of ECM neuroplasticity. These facilitate creation of new neurons, neural cell migration and differentiation, and axonal growth and pathfinding. ECM molecules are critical in all phases of synapses—origination, consolidation, strengthening, maintenance and metaplasticity.
When calcium and other signaling inside the cell triggers the start of a synapse, the ECM molecules are critical factors in carrying this out. There are many complex ways that reelin, hyaluraonic acid, and tenascin are involved. Integrin sticks out of the membrane, and communicates deep into the neuron, regulating the NMDA neuroplasticity signaling to these other molecules.
ECM is critical in the second phase—the consolidation of the synapse. These structural changes occur, first, inside the axon and dendrite with alterations in scaffolding proteins such as actin and motors like myosin. Actin communicates with the ECM proteins through integrin, which straddles the inside and outside of the membrane. Back and forth communication with the ECM molecules stimulates actin to build different structures.
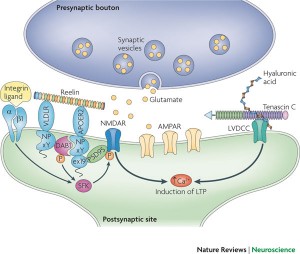 From inside the cell, calcium increase triggers synaptic plasticity. Reelin, sitting outside the cell, regulates the critical glutamate NMDA receptors. It aids migration of neurons and regulates the formation of the cortex and cerebellar structure by controlling the order of addition of cells to form the layers. Reelin continues to be a critical factor in the function of synapses.
From inside the cell, calcium increase triggers synaptic plasticity. Reelin, sitting outside the cell, regulates the critical glutamate NMDA receptors. It aids migration of neurons and regulates the formation of the cortex and cerebellar structure by controlling the order of addition of cells to form the layers. Reelin continues to be a critical factor in the function of synapses.
Many different ECM molecules are necessary for long term potentiation, LTP. LTP was the first type of neuroplasticity described at glutamate synapses in the hippocampus; it depends upon reelin, tenascin, chondroitin, and laminin.
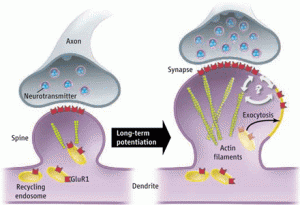 While sensory data and thoughts trigger rapid neuroplastic changes using ECM and actin, ordinary maintenance of the synapse occurs more slowly. ECM and actin are necessary to keep the appropriate level of signal, waiting for new signals with future changes. ECM molecules adjust the synaptic strength for maintenance. These available structures are critical for stabilization and future metaplasticity. In this process, newly discovered metallic enzymes cut ECM molecules allowing previously hidden binding sites on the integrin molecule, which opens different types of communication with the cell.
While sensory data and thoughts trigger rapid neuroplastic changes using ECM and actin, ordinary maintenance of the synapse occurs more slowly. ECM and actin are necessary to keep the appropriate level of signal, waiting for new signals with future changes. ECM molecules adjust the synaptic strength for maintenance. These available structures are critical for stabilization and future metaplasticity. In this process, newly discovered metallic enzymes cut ECM molecules allowing previously hidden binding sites on the integrin molecule, which opens different types of communication with the cell.
New synaptic configurations are triggered by neuroplasticity. Changes occur in the actin scaffolding structures, synthesis of many new proteins and strengthening the synapse. The ECM is involved in holding the two neurons and astrocyte more tightly. Alterations occur in dendrite spines, and the postsynaptic density. Integrin is critical in this process. Integrin is a critical receptor on the membrane of the cell. Integrin is connected to cascades that go deep into the cell and help form the glutamate subunits GluNsA and 2B (see post on glutamate neuroplasticity).
The ECM, also, provides molecules that increase the health of neurons—neurotrophins. They stimulate the outgrowth of dendrites and of buds from axons. The unique composition of the matrix provides added protection against invading tumors and microbes.
Proteoglycans and CSPG
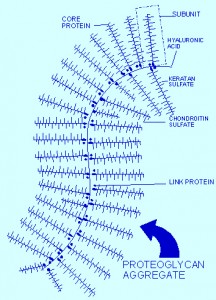 Proteoglycans are a central protein family consisting of various carbohydrate chains connected to a large protein. The side chains are negatively charged disaccharides, which determine three different categories of molecules. The most important molecule in this class is chondroitin sulfate proteoglycan (CSPGs), which consist of a protein core and a chondroitin sulfate side chain. HSPG is another important proteoglycan in Alzheimer’s.
Proteoglycans are a central protein family consisting of various carbohydrate chains connected to a large protein. The side chains are negatively charged disaccharides, which determine three different categories of molecules. The most important molecule in this class is chondroitin sulfate proteoglycan (CSPGs), which consist of a protein core and a chondroitin sulfate side chain. HSPG is another important proteoglycan in Alzheimer’s.
CSPG is made inside cells by a series of enzymes, which create the protein and attach carbohydrates, or glycans, to it. This molecule is then secreted from the cell and forms large structures. It is a complex process that includes connecting the tetra-saccharide bridge with other molecules and a large protein center.
GSPGs can block structural plasticity or allow it. They maintain structure where plasticity would be detrimental. GSPGs are involved in stability of memories in the amygdala, and help for perineural nets to maintain stability. Another molecule, neurotrypsin, from neurons in the cortex, hippocampus and amygdala combine with CSPGs and are necessary for higher brain function.
ECM Proteins Critical in Disease
Cancer: With some tumors, such as glioma, there is an abnormal increase in some of the constituent ECM molecules, which increase the tumor growth. Other components of the ECM can help or suppress the immune attack on these cancer cells.
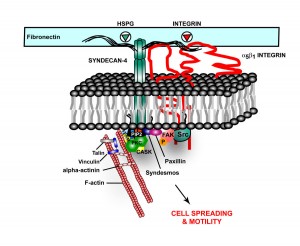 Alzheimer’s: In Alzheimer’s disease, HSPG, one of the proteoglycans, is part of the amyloid plaques and the neurofibrillary tangles. They appear to increase the formation of amyloid beta by stopping the enzymatic cleavage of the amyloid peptide and also interacting with apolipoprotein E (APOE), which is known as a major risk factor for Alzheimer’s. APOE helps transport lipid in the brain and helps with repair of neurons. The lipoprotein molecules include lipids that are used for the maintenance of neurons. When APOE4 cannot bind to HSPG then the lipoprotein function is impaired.
Alzheimer’s: In Alzheimer’s disease, HSPG, one of the proteoglycans, is part of the amyloid plaques and the neurofibrillary tangles. They appear to increase the formation of amyloid beta by stopping the enzymatic cleavage of the amyloid peptide and also interacting with apolipoprotein E (APOE), which is known as a major risk factor for Alzheimer’s. APOE helps transport lipid in the brain and helps with repair of neurons. The lipoprotein molecules include lipids that are used for the maintenance of neurons. When APOE4 cannot bind to HSPG then the lipoprotein function is impaired.
Brain Trauma: After brain trauma, especially spinal injury, there are specific interstitial changes. Scar formation includes large amounts of CSPGs mostly from astrocytes. Axons cannot cross these deposits, and form retraction bulbs, and even those that can traverse the scar have reduced connections. Specific types of GSPG are the most inhibiting of neuron growth.
Demyelination, Multiple Sclerosis and ECM Molecule CSPG
Trauma and degenerative disorders often include loss of myelin, and 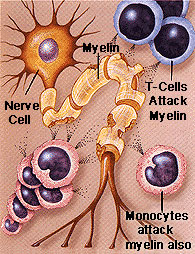 oligodendrocytes, the cells producing myelin.
oligodendrocytes, the cells producing myelin.
In multiple sclerosis, an autoimmune disease where immune factors destroy myelin, there are abnormal spots of basement membranes along with the presence of many immune cells. The membranes seem to attract leukocytes. It is known that perineural nets are eliminated near the neurons, leaving them unprotected. Also, neurons accumulate abnormal filaments inside.
Critical in this process is the increase of CSPG, which also occurs with CNS injury. In multiple sclerosis, GSPGs surround the edge of the lesions. These are incorporated into abnormal interstitial matrix. MS has increased CSPGs and hyaluronan deposited near the lesions that eliminate myelin. The physical blockage of the large hyaluronan molecule appears to be able to stop the creation of new oligodendrocytes and astrocytes. These ECM molecules both stimulate inflammation and also physically block myelination.
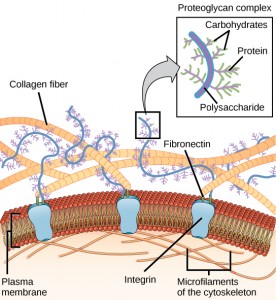 ECM molecular alterations affect all aspects of oligodendrocyte development, their duplication, migration, and myelination. Ordinarily the normal parts of the ECM like laminins and integrins help oligodendrocytes by stimulating signaling cascades. People without these laminins have defective myelination.
ECM molecular alterations affect all aspects of oligodendrocyte development, their duplication, migration, and myelination. Ordinarily the normal parts of the ECM like laminins and integrins help oligodendrocytes by stimulating signaling cascades. People without these laminins have defective myelination.
Fibronectin does the opposite. It stops differentiation of oligodendrocytes from stem cells, as well as impairing myelination. Tenascin can be helpful, R type, and unhelpful, C type.
CSPGs are the most powerful inhibitors of oligodendrocytes. This effect is dependent on myosin motors (see post on myosin neuroplasticity). CSPG also stop the oligodendrotcytes from migrating and the remyelination process.
Enzymes Constantly Remodel ECM Proteins
ECM is constantly changing, like all other aspects of the brain. It is difficult to determine how active remodeling can occur outside of cells. Where is the direction for these enzymatic actions? Cytokine signals direct immune cells. But, what is the signal that directs strands of protein in space?
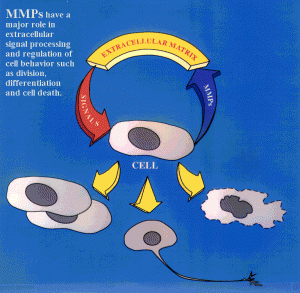 Specific enzymes are constantly remodeling the ECM. MMPs are a major class of protein enzymes (24 different versions) that all cut the ECM proteins. When these cuts are made signals are sent to the cells that influence many processes, especially the differentiation of stem cells and cell death pathways. In many diseases like cancer, stroke, MS, and spinal injury there is an increase in MMPs. These abnormal MMPs can kill cells, break the blood brain barriers and stimulate inflammation.
Specific enzymes are constantly remodeling the ECM. MMPs are a major class of protein enzymes (24 different versions) that all cut the ECM proteins. When these cuts are made signals are sent to the cells that influence many processes, especially the differentiation of stem cells and cell death pathways. In many diseases like cancer, stroke, MS, and spinal injury there is an increase in MMPs. These abnormal MMPs can kill cells, break the blood brain barriers and stimulate inflammation.
Strangely, they, also, help repair damage just after brain injury with increases in MMP2 related to reduced scarring, reduced neuronal cell loss and better improvement.
MMPs affect the local environment in the ECM during myelination and remyelination after injury. They cut laminins, CSPGs and integrin receptors. One particular enzyme MMP9 is needed for remyelination and clears CSPGs.
Extra Cellular Matrix Is Critical to Neuroplasticity
Just as there are significant electrical events in the regions outside of neurons and astrocytes (see post Electricity in the Brain), the extracellular matrix has critical functions for all aspects of neuroplasticity. Any changes in the three regions of the ECM—the basement membrane, the perineuronal networks, and the interstitial matrix—have drastic consequences.
Thought and experience determine the instantaneous molecular changes in widespread neuroplastic circuits. Thoughts and experiences trigger gene networks (see post), as if they were brains, stimulating the creation of new molecules. But, how do they determine the actions in regions where there are no cells? The ECM, responding to thought with neuroplasticity, consists of some attached and many free active molecules and enzymes outside of cells.
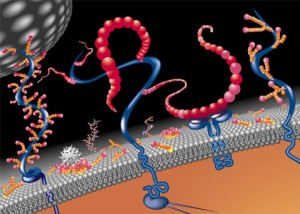 ECM is constantly changing, like all other aspects of the brain. One example is that perineural nets regulate specific windows of opportunity for plasticity in fetuses and children. Where is the direction for this? It is difficult to determine how active remodeling can occur outside of cells.
ECM is constantly changing, like all other aspects of the brain. One example is that perineural nets regulate specific windows of opportunity for plasticity in fetuses and children. Where is the direction for this? It is difficult to determine how active remodeling can occur outside of cells.
With such an active extracellular space, it is difficult to understand where the direction for the thousands of critical actions resides. When the integrin molecule communicates through the membrane, it is possible to understand direction coming from inside the cell through signaling cascades. Also, MMP enzymes cut ECM molecules which send signals to cells. But, most of the critical activity in the ECM occurs independent of direct connections with the neurons, astrocytes or microglia that produce the ECM matrix.
With so many mechanisms occurring simultaneously during neuroplasticity in wide brain circuits, it is reasonable to consider the mind providing the direction by interacting in all places at once. But, it is more difficult to consider the direction to complex, critical brain functions that occur with free-floating molecules in the ECM. Does the mind direct these as well?