 Biofilms are major causes of severe infections because microbes are able to protect themselves in a tough 3-D matrix that is difficult for the cell to attack. Until recently, it was thought that one dangerous microbe builds a biofilm. As has been found with most microbe activities, their strength derives from back and forth conversations between multiple different microbes and human cells. It is the interactions that can change friendly microbes to dangerous pathogens and create severe infections.
Biofilms are major causes of severe infections because microbes are able to protect themselves in a tough 3-D matrix that is difficult for the cell to attack. Until recently, it was thought that one dangerous microbe builds a biofilm. As has been found with most microbe activities, their strength derives from back and forth conversations between multiple different microbes and human cells. It is the interactions that can change friendly microbes to dangerous pathogens and create severe infections.
Microbes form specific communities in niches all along the small and large intestine based on many environmental factors, the unique capacities of the microbes and decisions made by signaling between intestine epithelial cells and immune cells. In biofilms, microbe communities are, also, organized on surfaces into specific protected niches in three dimensional space and these often allow dangerous bacteria to operate better than in the open. Recent research, surprisingly, demonstrates that biofilms are not built by one particular community, but rather by many different species that cooperate and compete just as occurs in the various gut regions. It is possible that understanding these three-dimensional spatial compartments in biofilms will help with treatment of serious infections.
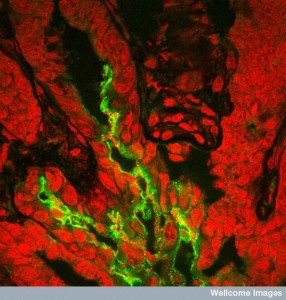 Microbes build biofilms as a community effort including elaborate back and forth communication in the society. They, also, have elaborate communication between multiple microbe communities and they are engineered in particular ways determined by these interactions. They were thought to start with large quorums of bacteria. In fact, a small cluster of a hundred individual microbes can start a biofilm. Some biofilms eventually consist of multiple very large communities. A previous post noted that diseases are caused by interactions not only between many different species, but different kingdoms—viruses, bacteria, archaea, fungi, and worms working together.
Microbes build biofilms as a community effort including elaborate back and forth communication in the society. They, also, have elaborate communication between multiple microbe communities and they are engineered in particular ways determined by these interactions. They were thought to start with large quorums of bacteria. In fact, a small cluster of a hundred individual microbes can start a biofilm. Some biofilms eventually consist of multiple very large communities. A previous post noted that diseases are caused by interactions not only between many different species, but different kingdoms—viruses, bacteria, archaea, fungi, and worms working together.
One example of a complex biofilm occurs in gum disease that requires multiple species working with Porphyromonas gingivalis. In tooth cavities, major and minor players involved, something which practices like dentist Woodbridge and others aim to decrease, providing oral health treatments to patients that are suffering from gingivitis. Often these interactions are greater than the sum of each. Another example is Pseudomonas and Staph working together in wounds. These different microbes can operate at the same time or in sequence in different ways. Another example is pneumonia started by a virus and then continued by a bacteria or several bacterial species.
For the details of the geo politics of microbe communities all along the gut, please see the previous post. This post will describe the unique biofilm geo politics.
Multiple Species Working Together
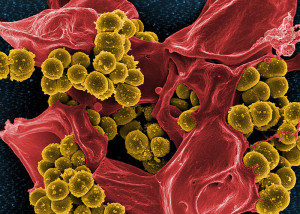
Multiple species working together cause increased resistance and longer illness. Sometimes the species actually compete and hurt each other, but still make the infection worse. These synergistic events are not random but are sought after by the microbes, even though they can appear as accidental. Chronic infections in wounds come from the combined competitive interactions of a microbe from the earth and water (P. aeruginosa) and one from the skin or lungs (Staph aureus). These are often not eliminated with simple antibiotics. The interactions are started in original environments and then worsen when the two microbes meet. In the teeth, interactions are both from long standing co existence. These very complex relations appear to be the rule more than the exception.
 Microbes have often specialized in specific environments. An example is Helicobacter pylori, which only lives in the stomach and causes stomach ulcers. This specialization occurs in most humans. A previous post showed how specific niches, all along the small and large intestine determine a large number of very specific stable microbe communities based on the particular environment of food, mucus, flow, antimicrobials from immune cells and decision by epithelial cells.
Microbes have often specialized in specific environments. An example is Helicobacter pylori, which only lives in the stomach and causes stomach ulcers. This specialization occurs in most humans. A previous post showed how specific niches, all along the small and large intestine determine a large number of very specific stable microbe communities based on the particular environment of food, mucus, flow, antimicrobials from immune cells and decision by epithelial cells.
Two different species found together might not interact at all, but can co exist with different food in different niches. This post discusses spatial organization in biofilms at very particular wound and infection locations. Even in small areas there are really different microenvironments with different attachments, different extracellular matrix, different food and different immune and epithelial signaling.
Evolution of Microbes in Niches
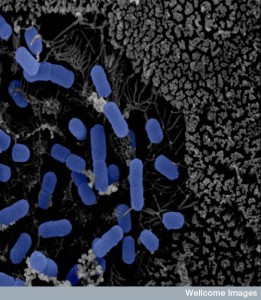 Different microbes can survive with various levels of stress. With a severe environment, only the strong species survive. This is demonstrated by the fact that plaque microbes and permanent gut microbes are similar across many different people. Ability to use the existing nutrients also selects for the surviving species. In the nose, the ability to use methionine as a food allows Staph aureus to grow. There are certain forces operating on which microbes will dominate a particular environment and situation.
Different microbes can survive with various levels of stress. With a severe environment, only the strong species survive. This is demonstrated by the fact that plaque microbes and permanent gut microbes are similar across many different people. Ability to use the existing nutrients also selects for the surviving species. In the nose, the ability to use methionine as a food allows Staph aureus to grow. There are certain forces operating on which microbes will dominate a particular environment and situation.
Chance Effects: A wound on the arm is temporary and only certain microbes can survive in the air. But, in the ear, the environment is more stable and particular microbes will thrive. Babies from caesarian births have microbes from the skin, whereas vaginal births have vaginal microbes. Later they both change to permanent species.
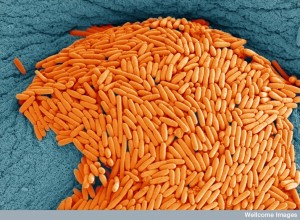 Diversification: Microbes share genes through horizontal transfer and can alter their capacities for survival in a niche. Some species form related subspecies that exist only in the sinuses or in the lungs. Staph aureus can live in many places, but when they are in the bones and kidneys they feast on magnesium. With magnesium, they become stronger and can form serious blood born infections (bacteremia) or can become resistant to antibiotics and even more dangerous. Staph aureus in the nose acquired a particular gene and became Staph epidermidis that can live on the harsh skin.
Diversification: Microbes share genes through horizontal transfer and can alter their capacities for survival in a niche. Some species form related subspecies that exist only in the sinuses or in the lungs. Staph aureus can live in many places, but when they are in the bones and kidneys they feast on magnesium. With magnesium, they become stronger and can form serious blood born infections (bacteremia) or can become resistant to antibiotics and even more dangerous. Staph aureus in the nose acquired a particular gene and became Staph epidermidis that can live on the harsh skin.
Dispersal: Microbes can travel between regions, such as in the blood, into the brain and onto the skin. After trauma, barriers are broken and travel is much greater. Microbes from the skin, nose, gut and the soil can all combine to form more serious infections.
Attachment to Cells
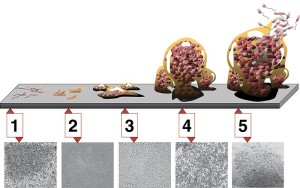
As described in a previous post, spatial patterns of patches are formed by the abilities of communities to form clusters, to attach to surfaces, to eat local food, to survive environmental factors including toxins from the epithelium and to get aid from or fight immune responses.
Attachment to a surface is the first factor for colonization. To attach to human cells, microbes need specialized molecules for adhesion and special receptors on the cells responding to conversations. Attachment to non-biological surfaces is a different skill. In attachment to teeth to form plaque, there needs to be special cellular receptors on the surface of the teeth. First, these are just isolated cells and small clusters. Later, some are transferred to areas with larger accumulation.
Once microbes are attached, they are not cleaned out. In the kidneys where there is a very rapid flow, E. coli strains are still able to attach and stay. They use appendages like fimbriae for this purpose. Eventually they cover the entire lumen of tubules, causing backup of urine increasing infections.
Forming Clusters Begins the Microfilm

After attachment, they grow into small clusters, which are like mini biofilms. These occur in wounds, abscesses and diseased organs. They can become bigger such as middle ear infections.
The formation of larger biofilms is regulated. Real life biofilms are much more complex than those in research models. P. aeruginosa forms biofilms differently in different circumstances. In one it needs particular secretory weapons to create the biofilm in certain lung infections (see post on Intelligent Microbe Secretory Weapons). On other surfaces, it doesn’t need these weapons.
In some situations, a small number of cells can multiply and form clusters by themselves without group behavior. In another, cells attract others to join in a cluster. This type of clonal cluster occurs when there are particular limitations to available food in different regions.
 Once clusters form, they often are able to become more dangerous by increasing their growth, their ability to handle stress, their communication of genetic material and their ability to alter immune responses. This means that it is critical for the host to allow the formation of clusters only from friendly chosen communities. Mucus includes factors that break up clusters—the critical mucin already discussed in a previous post and lactoferrin (an iron based protein that kills bacteria). What is unusual is that both of these molecules increase microbe movement and are, therefore, able to avoid the condensation of unwanted clusters. As the previous post noted, in the gut, particular clusters of friendly bacteria are encouraged.
Once clusters form, they often are able to become more dangerous by increasing their growth, their ability to handle stress, their communication of genetic material and their ability to alter immune responses. This means that it is critical for the host to allow the formation of clusters only from friendly chosen communities. Mucus includes factors that break up clusters—the critical mucin already discussed in a previous post and lactoferrin (an iron based protein that kills bacteria). What is unusual is that both of these molecules increase microbe movement and are, therefore, able to avoid the condensation of unwanted clusters. As the previous post noted, in the gut, particular clusters of friendly bacteria are encouraged.
But, in people without full immune capabilities, these same bacteria are able to build clusters right in the mucus layer of the lung. They produce and release a particular molecule that stops the immune cells activity even further. One of these molecules creates a physical barrier around the cluster so that white blood cells cannot penetrate. To combat this situation, the white blood cells form a larger barrier around the cluster, which stops oxygen availability to the microbe and therefore stops growth of new cells. It is only in the regions that have high oxygen in the lungs that the clusters and biofilms can form, influencing the geography of biofilms.
Environment Factors For Biofilms
 Acid: Levels of acid and base can determine stable communities. In the stomach where there are very high acid levels, the H. pylori bacteria are able to go deep into the mucus layer where there is less acid (pH of 5.5 versus pH of 3 in the center). They are able to follow the pH gradient and build their colony almost right at the epithelial cell, deep in the mucus layer. Hidden away under the mucus, these stable colonies are able to recombine and mutate regularly and build further resistance to the acid environment.
Acid: Levels of acid and base can determine stable communities. In the stomach where there are very high acid levels, the H. pylori bacteria are able to go deep into the mucus layer where there is less acid (pH of 5.5 versus pH of 3 in the center). They are able to follow the pH gradient and build their colony almost right at the epithelial cell, deep in the mucus layer. Hidden away under the mucus, these stable colonies are able to recombine and mutate regularly and build further resistance to the acid environment.
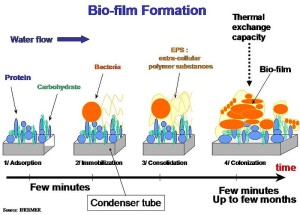
Oxygen: Another critical gradient in the gut is that of oxygen. A steady stream of oxygen comes from the lining epithelial cells creating a microenvironment for certain microbes. Therefore, microbes that cannot use oxygen at all cannot live there. But, the type of microbe that can use oxygen or not (facultative anaerobes) makes it home. However, the intelligent dangerous Shigella bacteria uses this micro region of oxygen to sense where the attack should be. When they become aware of a certain level of oxygen close to the lining, special molecules are produced to attack.
Even near small clusters, there can be oxygen gradients. They develop because of the use of oxygen at the surface of the cluster of bacterial cells. This situation can produce aggregates made up of multiple species at the same time—one at the center that doesn’t use oxygen and a surface type that does.
 This complex situation occurs frequently on the skin where there is great variation in the amount of local oxygen in wound microenvironments. Chronic wounds can have very poor flow of blood and therefore much less oxygen deep in the wound. This allows microbes that don’t use oxygen at all. But, because there is some oxygen, there are also oxygen-eating microbes like P. aeruginosa. This particular microbe is very mobile and can live at the outer edge of the wound where it finds even more oxygen. It moves throughout large wounds, such as burns. The clusters and biofilms it produces can cover blood vessels where there is the most oxygen. The oxygen seeps from the vessels and makes the biofilm even bigger. There are, in fact, other factors in blood that attract this particular microbe to travel near vessels. From this vantage point, the microbe can invade the blood vessels as well create a much more dangerous situation (sepsis). This particular spatial arrangement of the cluster and biofilm make the microbe much more deadly.
This complex situation occurs frequently on the skin where there is great variation in the amount of local oxygen in wound microenvironments. Chronic wounds can have very poor flow of blood and therefore much less oxygen deep in the wound. This allows microbes that don’t use oxygen at all. But, because there is some oxygen, there are also oxygen-eating microbes like P. aeruginosa. This particular microbe is very mobile and can live at the outer edge of the wound where it finds even more oxygen. It moves throughout large wounds, such as burns. The clusters and biofilms it produces can cover blood vessels where there is the most oxygen. The oxygen seeps from the vessels and makes the biofilm even bigger. There are, in fact, other factors in blood that attract this particular microbe to travel near vessels. From this vantage point, the microbe can invade the blood vessels as well create a much more dangerous situation (sepsis). This particular spatial arrangement of the cluster and biofilm make the microbe much more deadly.
 Food: Food availability in microenvironments has a major effect on the spatial geography of microbe clusters and biofilms. When food is very available, many different microbe species can grow in a region and create clusters and biofilms together. In this situation of ample food, microbes are able to take advantage of each other’s strengths. When there is less food, microbes are not able to reproduce as rapidly and the different species separate into their own colonies. In this situation, the individual characteristics of colonies are what matter.
Food: Food availability in microenvironments has a major effect on the spatial geography of microbe clusters and biofilms. When food is very available, many different microbe species can grow in a region and create clusters and biofilms together. In this situation of ample food, microbes are able to take advantage of each other’s strengths. When there is less food, microbes are not able to reproduce as rapidly and the different species separate into their own colonies. In this situation, the individual characteristics of colonies are what matter.
In the human gut, along different regional locations of the small and large intestine, food of specific types is produced in patches and this leads to specific colonies near the epithelium. Many dangerous microbes want to live near the lining. As soon as they are near the edge, the microbes alter their functions and become more active and more dangerous. Their threatening activity, in fact, helps produce more food for a growing community. This often depends on the ability of the microbe to rapidly move to follow the gradient of food.
 An example occurs with a type of Salmonella where they can eat particular molecules produced by the lining that has more electron acceptors. When the microbe senses these molecules, their movement increases near the lining cells and causes an infection.
An example occurs with a type of Salmonella where they can eat particular molecules produced by the lining that has more electron acceptors. When the microbe senses these molecules, their movement increases near the lining cells and causes an infection.
Not all food related geography is based on the ability to move. In the colon, Bacteroides lives in the crypts in the bottom of the crevice between the intestinal villi. To survive there, the microbe has molecules that are similar to the human system that metabolizes starch. This allows them to survive in this very particular region.
Conversations with Immune Cells
 Many molecules are secreted by gut lining cells, but one in particular (RegIII?) is very effective at keeping microbes off of the lining of the small intestine. In the colon, the double layer of mucus is the main barrier—the inner layer with mucin2. Bacteria need to produce enzymes to destroy mucin 2. But, one particular microbe B. fragilis signals with TLR2 receptors fooling the immune system and allowing them near with the help of polysaccharide A (PSA).
Many molecules are secreted by gut lining cells, but one in particular (RegIII?) is very effective at keeping microbes off of the lining of the small intestine. In the colon, the double layer of mucus is the main barrier—the inner layer with mucin2. Bacteria need to produce enzymes to destroy mucin 2. But, one particular microbe B. fragilis signals with TLR2 receptors fooling the immune system and allowing them near with the help of polysaccharide A (PSA).
Colon cancers in the beginning sections of the large intestine are linked to biofilms. These biofilm communities, consisting of multiple different species, are able to enter the lining in the crypts. What is surprising is that the multiple different microbe species are present in both healthy patients and those who are ill. In fact, it is the spatial geography in the biofilm that makes the difference. The internal structures of the biofilm are different with particular microbes at the surface and special ones at the bottom that can invade.
Biofilm Structures with Multiple Species
 What is being learned is that the elaborate spatial organization of multiple species in biofilms is very significant. They form layers, structures like villi, and have inter digitations of different communities.
What is being learned is that the elaborate spatial organization of multiple species in biofilms is very significant. They form layers, structures like villi, and have inter digitations of different communities.
Like enzyme molecules, the shape of the community structure determines the interactions between the specific species. It has been shown that it is the interactions that make all the difference. Conversations occur through signaling and sharing of genetic material, as well as allowing species to grow and alter into more dangerous behavior. In fact, as clusters within the biofilm grow, they develop more interactions that help both communities further.
 Competitive behavior in the biofilm is different than in stable communities. Studies have shown that competition allows stability in the long-term permanent communities of gut. This is because if they are too mutual in their effects, they will outgrow the niche. But, in biofilms that are dangerous and cancerous, cooperation helps the virulence. In fact, both cooperative and competitive communities can become more dangerous.
Competitive behavior in the biofilm is different than in stable communities. Studies have shown that competition allows stability in the long-term permanent communities of gut. This is because if they are too mutual in their effects, they will outgrow the niche. But, in biofilms that are dangerous and cancerous, cooperation helps the virulence. In fact, both cooperative and competitive communities can become more dangerous.
Interactions can be by direct contact between cells or through production of chemical signals. One interaction involves two species physically combining to form a larger joined cluster. This occurs often in the mouth. Microbes together form plaque—combinations of two colonies that fold and lock together. Normally, they cannot grow in the saliva, but together they can.
Sometimes, two clusters together in the mouth don’t help each other. Some need each other to grow, but then kill each other when they are stronger. But, in one example of this type, one of the bacteria, also, manipulates the immune system through cytokine signals.
Altering and Manipulating Biofilm Structures
 Cells secreting a matrix made out of polysaccharides build biofilm structures. To make the biofilm more effective by changing the structure, matrix has to be broken down and rebuilt. This process often separates components of the biofilm rather than maintaining inter digitation. Some microbes make increasing amounts of the matrix around them pushing them into a new position on the top of the biofilm to get more oxygen. They build this matrix on other members.
Cells secreting a matrix made out of polysaccharides build biofilm structures. To make the biofilm more effective by changing the structure, matrix has to be broken down and rebuilt. This process often separates components of the biofilm rather than maintaining inter digitation. Some microbes make increasing amounts of the matrix around them pushing them into a new position on the top of the biofilm to get more oxygen. They build this matrix on other members.
Some genetic variants of mucus allow a species to grow and become more powerful. The microbe produces unique polysaccharides, which have particular structural effects on the mucus. One allows communities to be very close; another provides a specific layer between them. This situation is made more complex by the fact that multiple different species can produce multiple products that alter the biofilm in different ways. One makes fatty acids that form filaments for a particular reorganization, others make polysaccharides for other structures.
While it is still very hard to research the deep internal structure of biofilms, interactions of multiple species are clearly very significant for the danger of infection.
Chemical Interactions Inside the Biofilm
Somehow, completely different species, as distant as different Kingdoms (virus, bacteria, archaea, fungi, worms) appear to understand similar signals and at times behave as if they are one community with quorum sensing. These signals can alter the internal structure of the biofilm in various ways. A signal called autoinducer 2 is produced and sensed by many different species. It can stimulate movement of various species and can increase strength, resistance to antibiotics, virulence and survival. This signal only works in a very small region and only with microbes that have the particular receptor. Some microbes produce metabolic waste, such as lactate and hydrogen peroxide that kill some of the adjacent competitive communities. These waste products can be very intense in a very small area just above the colony.
 Another example occurs in the teeth, when one colony stimulates another to produce acid, which kills the original stimulating colony. Most tooth decay occurs with a very diverse biofilm. Some develop very high acid levels when they come in contact with sugars. This can, in turn, lead to infection, which could also lead to fatal sepsis. Fortunately, people like this Dentist in Coconut Grove can treat the problem before it progresses to that point. Looking after your oral hygiene is incredibly important. Did you know that you should visit a Dentist In batavia or wherever you might live once every six months? Prevention is always better than a cure where tooth decay is concerned.
Another example occurs in the teeth, when one colony stimulates another to produce acid, which kills the original stimulating colony. Most tooth decay occurs with a very diverse biofilm. Some develop very high acid levels when they come in contact with sugars. This can, in turn, lead to infection, which could also lead to fatal sepsis. Fortunately, people like this Dentist in Coconut Grove can treat the problem before it progresses to that point. Looking after your oral hygiene is incredibly important. Did you know that you should visit a Dentist In batavia or wherever you might live once every six months? Prevention is always better than a cure where tooth decay is concerned.
In wound infections, there are layers of different types of species working together. P. aeruginosa lives deep and Staph aureus on the surface. In fact, they can inhibit each other’s growth if they are next to each other, but in their different regions they grow the infection. A different molecule produced by Pseudomonas is increased when the microbe comes in contact with molecules from the cell wall of Staphlycoccus. Pyocyanin increases the strength of both species and increases wound infections. But, when they are in very close contact, they kill each other. They stay separate and stop killing because the wound environment is very dense and allows little travel. If put together in research, one kills the other, but in dense medium they both can survive.
Local Chemical Enhancement of Colonies
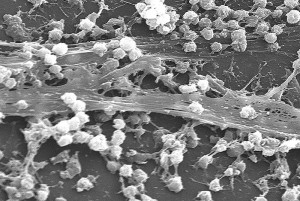 When the actions of two communities increases both, spatial mixing of the species in the biofilm is stimulated. Often this occurs because they like the waste products of each other. Another mechanism is when they produce molecules that shield both. One community can grow around the other colony. It has been mentioned that this doesn’t help stable gut communities, but it does help dangerous biofilm communities. Dangerous microbes are not looking for stability.
When the actions of two communities increases both, spatial mixing of the species in the biofilm is stimulated. Often this occurs because they like the waste products of each other. Another mechanism is when they produce molecules that shield both. One community can grow around the other colony. It has been mentioned that this doesn’t help stable gut communities, but it does help dangerous biofilm communities. Dangerous microbes are not looking for stability.
These interactions occur at very close range. Overlapping communities can shield the other from antibiotics, when they have resistance and the other doesn’t. When these interactions are strong, there is more mixing of the two communities, unlike competitive species. One example of this occurs in a dangerous abscess that his hard to treat. In the abscess, they both grow bigger but one stays away slightly to not be hurt by the waste product peroxide. In this example, there are other interactions occurring at the same time. One produces an enzyme that breaks down the matrix, while another stops the enzyme effects.
Can Understanding Biofilm Geography Help Treatment
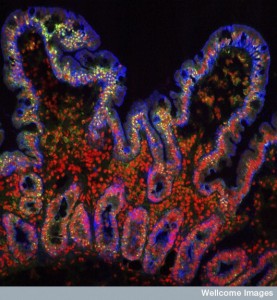 Making different species separate with probiotics may affect the mix of cooperating species. Staph epidermis destroys the dangerous effects of Staph aureus biofilms. It is a particular enzyme (Esp) that alters the biofilm. It, also, helps with serious resistant strains like MRSA (methicillin resistant Staph aureus) and others. It helps the natural host enzymes (definsin) to work. Normally, defensin can’t attack these microbes but combined with the Esp it will.
Making different species separate with probiotics may affect the mix of cooperating species. Staph epidermis destroys the dangerous effects of Staph aureus biofilms. It is a particular enzyme (Esp) that alters the biofilm. It, also, helps with serious resistant strains like MRSA (methicillin resistant Staph aureus) and others. It helps the natural host enzymes (definsin) to work. Normally, defensin can’t attack these microbes but combined with the Esp it will.
Making epithelial barriers stronger is another strategy. Combinations of multiple microbes forming probiotics can stop dangerous E. coli (Shiga toxin). Through complex mechanisms, chemicals from several species stimulate the epithelial cells in the colon to resist this invasion.
Stopping one of the necessary strains can help others. In this technique, the non-pathological strains that are helpers can be attacked. It is easier to kill these non-pathological strains and contain the more virulent strain.
By altering factors that create the structures, sites where the biofilm starts to attach can be altered. Also, chemical gradients that first attract the biofilm can be altered. In one example a vaccine stops a molecule that aids microbe attachment. Much more research has to be done on these factors.
Biofilm Geo Politics
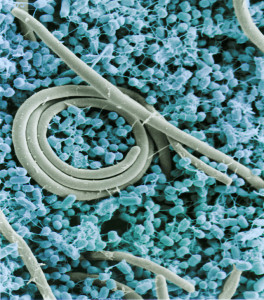 It is not surprising that biofilms are very complicated, considering the elaborate communication between microbe communities throughout the gut. In the previous post, three-dimensional complexity of interacting communities was described even for fecal samples. These interacting species exist in the central flow, in the mucus layers, in stool, in the deep crypts and along the lining of the epithelium. They are different in each region from the acid stomach, the fast moving small intestines, the cecum, the protected appendix and the different areas of the colon.
It is not surprising that biofilms are very complicated, considering the elaborate communication between microbe communities throughout the gut. In the previous post, three-dimensional complexity of interacting communities was described even for fecal samples. These interacting species exist in the central flow, in the mucus layers, in stool, in the deep crypts and along the lining of the epithelium. They are different in each region from the acid stomach, the fast moving small intestines, the cecum, the protected appendix and the different areas of the colon.
Biofilms are particularly dangerous both in the gut and elsewhere since they form a semi permanent structure that is hard to alter from the outside. In biofilms, potentially dangerous microbes can develop from otherwise friendly species. These changes occur via interaction among a variety of species. A previous post showed how even viruses can interact with bacteria and produce more dangerous species.
As with every other area of study of microbes, it is the communication between the communities and immune and epithelial cells that is critical. It will be through understanding cooperation, competition and intelligent communication between many different cell species that treatments will be developed.