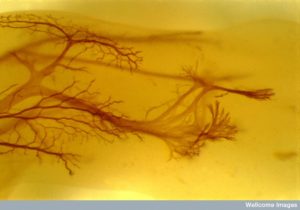
 Dendrites have been considered passive calculators of input signals. In fact, they are extremely dynamic and can produce their own electrical spikes. Dendrites have a vast array of different ways to function when helping to determine the next axon action potential. Recent research has begun to scratch the surface of the complexity of dendrite calculations. A previous post described details about the ever changing and varied dendrite spines and necks with many unique shapes that affect electrical and chemical properties.
Dendrites have been considered passive calculators of input signals. In fact, they are extremely dynamic and can produce their own electrical spikes. Dendrites have a vast array of different ways to function when helping to determine the next axon action potential. Recent research has begun to scratch the surface of the complexity of dendrite calculations. A previous post described details about the ever changing and varied dendrite spines and necks with many unique shapes that affect electrical and chemical properties.
Very recently it has been determined that there are at least two interacting processes that determine the action potential, each with a large number of variations. One is dendrites producing their own electrical spikes that travel to the cell body and axon hillock. The other is backward flow of electricity from the action potential through the cell body to the dendrite tree, where it mingles with the signals coming from the dendrites. This post will describe the latest understanding of the extremely complex ways that decisions are made in dendrites using many versions of these two signals.
First, the next section provides a brief summary of material from the previous post about dendrites, Vast Complexity of Dendrite Function.
Summary of Previous Dendrite Complexity Post
 Dendrites have multiple specific compartments with different mechanical and electrical properties that interact with the cell body (soma) and the axon. The dendrite, its spines and heads are different compartments with molecules travelling back and forth between them. Most proteins are made in the larger dendrite and transported to the spines and heads. Also, multiple spines can together form a unique compartment.
Dendrites have multiple specific compartments with different mechanical and electrical properties that interact with the cell body (soma) and the axon. The dendrite, its spines and heads are different compartments with molecules travelling back and forth between them. Most proteins are made in the larger dendrite and transported to the spines and heads. Also, multiple spines can together form a unique compartment.
Dendrites behave like amoebas producing filopidia that become spines. Spines grow on dendrites, sometimes suddenly; they attract axons and form synapses. Spines have unique shapes and varied geometrical placement. A strong signal to one spine can immediately cause others to form around it—to connect to that same neuron. Spines can be thin, stubby and mushroom shaped and are altered rapidly by neuroplasticity.
Dendrites produce the enormously complex postsynaptic density (PSD) made of thousands of unique interacting proteins, which is the receiving area of neurotransmitters sent by the axon bouton active zones. PSD proteins are different in each brain region. There are many other unique proteins that regulate flow of electricity and are constantly altering the actin scaffolding structures.
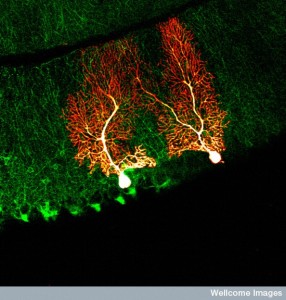 A dendrite tree can have hundreds of thousands of connections. Recent research finds that there are, in fact, 26 different sizes for synapses on spines, which greatly increases the possibilities of information utilization (previously only a small number of different sizes were known). The largest synapse is 60 times the smallest. When action potentials arrive at a dendrite, only 20% are activated. Dendrites change sizes based on activity—smallest dendrites increase it size with a thousand signals in 20 minutes and largest with hundreds in 2 minutes. Computation can occur in one single spine or in multiple spines that can be in different geometric configurations both near each other and at great distance in the dendrite arbor. A strong signal can affect an entire branch of the tree with a large number of dendrites.
A dendrite tree can have hundreds of thousands of connections. Recent research finds that there are, in fact, 26 different sizes for synapses on spines, which greatly increases the possibilities of information utilization (previously only a small number of different sizes were known). The largest synapse is 60 times the smallest. When action potentials arrive at a dendrite, only 20% are activated. Dendrites change sizes based on activity—smallest dendrites increase it size with a thousand signals in 20 minutes and largest with hundreds in 2 minutes. Computation can occur in one single spine or in multiple spines that can be in different geometric configurations both near each other and at great distance in the dendrite arbor. A strong signal can affect an entire branch of the tree with a large number of dendrites.
Most spines have a bulbous heads and thin necks connecting to the shaft of the dendrite, but their shape and functions change rapidly from many factors: BDNF, environment, seasonal change, age and estrogen, stress affects.
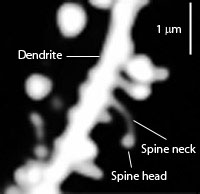 Spines stick out from every part of the dendrite. The neck is very small—a tenth of the diameter of a small bacterium. The head and the neck are different compartments with different electrical and chemical properties and unique ion channels. Unique shapes inhibit electricity in different ways.
Spines stick out from every part of the dendrite. The neck is very small—a tenth of the diameter of a small bacterium. The head and the neck are different compartments with different electrical and chemical properties and unique ion channels. Unique shapes inhibit electricity in different ways.
Neuroplasticity can alter one spine, multiple spines, the entire dendrite and the neuron cell body. Alterations in the spine head occur and then proteins are triggered to maintain these changed structures. Proteins can be made quickly nearby or slowly through signals to the cell nucleus. Necks can create resistance to electricity from the head and synapse and this resistance can be greater with a stronger signal. Necks can suddenly grow 40 times larger. Necks and heads are regulated independently. Neuroplasticity can permanently lower this resistance.
There are many other structural considerations. A short wide neck allows easier travel of materials between neck and head. Special rings in the neck stop the wrong proteins from entering. Special proteins span the membranes to the internal actin and shape the neck and head. Neuroplasticity has dramatic effects on the scaffolding molecules that affect shapes.
The spine is tiny compared to the nucleus, but can trigger genetic networks producing unique proteins. Proteins made in the nucleus are tagged for particular dendrites that grab them as they pass by.
Dendrite Decision Making
 Typically, dendrites receive thousands of signals at the same time. A calculation of some sort from these many inputs triggers another message. Research has found that there are many different ways that the information is integrated in a single neuron. The shape of the dendritic spines, the many variations of geometrical inputs and different active and passive properties all contribute to the outcome.
Typically, dendrites receive thousands of signals at the same time. A calculation of some sort from these many inputs triggers another message. Research has found that there are many different ways that the information is integrated in a single neuron. The shape of the dendritic spines, the many variations of geometrical inputs and different active and passive properties all contribute to the outcome.
Early experiments discovered dendrite properties in cell cultures and these have recently been found in the living brain. While it was thought that dendrites determine the axon action potential, it has only recently been demonstrated in the living brain. At first, research showed different dendrite activity altering and filtering electricity that travels to the axon initial segment (AIS is the first part of the axon leading from the soma or cell body of the neuron). More recently the full complexity of dendrite spikes interacting with backward signals from the axon hillock are being found.

Specific physical properties of the dendrites have important effects on the signals. Unique membranes in the dendrites affect the electrical resistance (the difficulty a charge has in passing through) and capacitance (the ability to store a charge). Also, three-dimensional shapes make it possible for many very complex signals; these are not often simple linear summations.
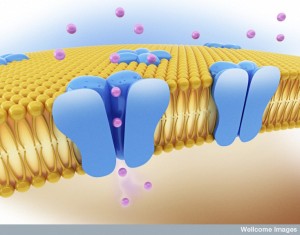
Dendrites have ion channels that are similar to axons, dependent on electrical charge and voltage. Dendrites now are known to contribute their own electrical charge to the current. These are called dendritic spikes and travel from a dendrite into the extremely large, dense and complex dendritic arbor.
History of Dendrite Spikes
Because of the complexity of dendrite activity, historically, different findings were discovered and thought to be the only mechanisms. Recently, the vast complexity is being unearthed.
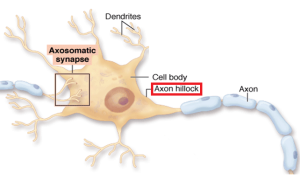 The earliest research with motor neurons in the spine implied that the electricity flowed passively backwards into the dendrites as well as forward from the AIS. Only recently have live measurements in the layers of the hippocampus shown that dendrites themselves actively fire their own many smaller currents. Purkinje neurons in the cerebellum and pyramidal neurons of the hippocampus also show dendrites making active currents. While in some cases passive electricity flowed backwards from the AIS to the soma to dendrites, in many cases, dendrites were active participants in firing and adding to the axon action potential.
The earliest research with motor neurons in the spine implied that the electricity flowed passively backwards into the dendrites as well as forward from the AIS. Only recently have live measurements in the layers of the hippocampus shown that dendrites themselves actively fire their own many smaller currents. Purkinje neurons in the cerebellum and pyramidal neurons of the hippocampus also show dendrites making active currents. While in some cases passive electricity flowed backwards from the AIS to the soma to dendrites, in many cases, dendrites were active participants in firing and adding to the axon action potential.
Later, live studies showed that when the intensity was low, the initial segment would start an action potential that flowed down the axon and it would, also, flow backwards to the dendritic tree influencing them. But, at high intensities, the dendrites themselves would produce spikes that travel to the AIS and influenced it.
 Then, multiple channels were found to be very active, such as calcium channels and sodium channels in the dendrites that respond to voltage. Two photon microscope cameras observed individual spines and found that dendrites are isolated electrical and chemical compartments each with their own properties independent of the axon. These studies show that while specific events can occur in the dendrite electrically, the current can change considerably by the time it reaches the AIS.
Then, multiple channels were found to be very active, such as calcium channels and sodium channels in the dendrites that respond to voltage. Two photon microscope cameras observed individual spines and found that dendrites are isolated electrical and chemical compartments each with their own properties independent of the axon. These studies show that while specific events can occur in the dendrite electrically, the current can change considerably by the time it reaches the AIS.
There are a wide range of complex ion channels in dendrites, including different kinds of sodium, potassium, calcium, and HCN (hyperpolarization activated cat-ion) channels. A previous post showed that there are hundreds of different types of potassium channels in neurons and vast complexity. Dendrite channels have many different sub types, with varied characteristics and very different spatial arrangements. All of this makes the electric system in dendrites vastly more complex than previously thought.
 One example is particular types of sodium channels in some dendrites, but not others. They are present in the cortex and hippocampus pyramidal cells but not in the cerebellum Purkinje cells. Another example is many different kinds of potassium channels all along the hippocampal dendrite arbors that are quite different when they are closer to the soma. Many of the channels are more densely arranged in the dendrites furthest from the soma. All of these differences make for extremely different complex electrical signals.
One example is particular types of sodium channels in some dendrites, but not others. They are present in the cortex and hippocampus pyramidal cells but not in the cerebellum Purkinje cells. Another example is many different kinds of potassium channels all along the hippocampal dendrite arbors that are quite different when they are closer to the soma. Many of the channels are more densely arranged in the dendrites furthest from the soma. All of these differences make for extremely different complex electrical signals.
Other recent findings shows that it is very common that the action potentials starting at the AIS send signals backwards that have a complex effect on the activity that is occurring in the dendrites from their inputs. In fact, it is the many different types of the channels in the dendrites that effect how the action potential of the AIS flows backwards and affects the dendrites.
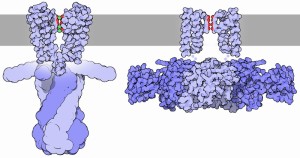 Many types of neurons have dendrites that create their own unique small action potentials. Sometimes, these have an influence on the AIS and the axon current and sometimes they don’t travel to the soma and axon based on the types of channels used. Even when the dendrite currents themselves don’t reach the soma and axon, they can have dramatic effects on the membranes of the soma and axon. These effects on the membranes can alter the timing of the action potential and even whether the spike will occur at all.
Many types of neurons have dendrites that create their own unique small action potentials. Sometimes, these have an influence on the AIS and the axon current and sometimes they don’t travel to the soma and axon based on the types of channels used. Even when the dendrite currents themselves don’t reach the soma and axon, they can have dramatic effects on the membranes of the soma and axon. These effects on the membranes can alter the timing of the action potential and even whether the spike will occur at all.
Many Kinds of Dendritic Spikes
 The large amount of recent research shows different, possibly contradictory, results. Different channel types create very different dendrite electrical spikes. One particular type of spike is from the dendrite sodium channels that are small in size and are completely independent of any back current from the AIS. The calcium channels are larger and produce a different type of calcium dendrite current that is longer. Another dendritic spike is produced by NMDA channels and is even longer. It is mostly produced by very particular isolated dendrites that are small (in the cortex they are called basal and tuft dendrites).
The large amount of recent research shows different, possibly contradictory, results. Different channel types create very different dendrite electrical spikes. One particular type of spike is from the dendrite sodium channels that are small in size and are completely independent of any back current from the AIS. The calcium channels are larger and produce a different type of calcium dendrite current that is longer. Another dendritic spike is produced by NMDA channels and is even longer. It is mostly produced by very particular isolated dendrites that are small (in the cortex they are called basal and tuft dendrites).
All of these spikes exist mostly in a particular compartment in the dendrite that is separated from the soma. There are many different types of signals based on the whether the compartment has different types of resistance and capacitance electrical properties. The three dimensional structure of the dendrite arbor influences the results. Some start in very small dendrites but then travel to a much larger arbor where the resistances is greater. Many of the spikes do not travel far, but some are able to have dramatic influence on the axon spike.
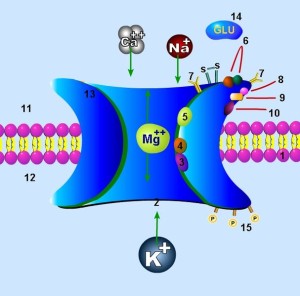
The calcium spikes are larger and more often influence the axon, particularly when the neuron is involved in high frequency pulsed signals. Some of the potassium channels produce spikes that slow the larger calcium spikes. The NMDA signals never travel very far since they require glutamate to trigger a larger response. But, they can strongly influence membranes that, in turn affect the membrane potentials at the AIS. Longer calcium and NMDA spikes have recently been found to be influential in neuroplasticity and tuning of the sensory neuron. They do this by affecting the AIS membranes, which increases the likelihood of an axon spike.
Research is just now uncovering the many ways that backward signals from the axon action potential and the small dendritic spikes interact. If the signal to the synapse and dendrite is not strong, then the backward spike can increase the calcium spike of the dendrite by changing the threshold. This can have a rebound effect of making a larger axon action potential. It can rebound and cause a burst of action potential firing as well. The opposite can occur. The backward electricity can wipe out the dendrite firing of sodium channels.
Dendrites Compute in Many Ways

The many variations described allow dendrites to make a wide range of different computations of electricity. One type of response is additive where multiple synchronized pulses hit one dendrite area causing a spike based on addition of the currents. This is the simple linear summation that was once considered to be the main process. These have to be very well synchronized.
Another type of additive event can occur when two signals hit at the same time, but in different parts of the dendrite tree. One hits a dendrite close to where the axon signal is coming from and the other hits closer to the cell body magnifying the first one. This addition is based on three-dimensional geometry of the arbor.
Another type of summation is when the passive backward electricity from the axon action potential combines with incoming signals to form a combined dendrite spike. And another occurs when there needs to be multiple large branches of the tree hit by currents at the same time.

Another type of response is not a sum, but occurs with competition where only one of the signals is chosen. This occurs when dendrites are set up to have enough power to cause an axon spike if multiple spines on the same dendrite are hit at once. Several of these pre formed dendrite situations compete to be the one that determines the axon action potential and the other is blocked.
There are more complex arrangements and interactions. One example is when addition occurs with several inputs and another inhibits some or all of them. This often happens when an early distant dendrite is hit by one or more signals and then a larger region closer to the soma hits and inhibits these.
All of these, and more, can be combined for very complex interactions from thousands of places on the tree at once. Real life dendrite activity is modulated by many special pathways, which can change the properties of regions of the dendrites.
In Live Animal Brains
 With optogenetics and other techniques such as patch clamps, dendrites are being more closely observed in the highly layered hippocampus. Recently, findings of backward passive electricity from axon to dendrites were confirmed in live brains using these techniques. Some of these studies are consistent with linear additive computations with visual, auditory and somatosensory inputs, similar to motor neurons. These can be complex additions but are often fairly linear.
With optogenetics and other techniques such as patch clamps, dendrites are being more closely observed in the highly layered hippocampus. Recently, findings of backward passive electricity from axon to dendrites were confirmed in live brains using these techniques. Some of these studies are consistent with linear additive computations with visual, auditory and somatosensory inputs, similar to motor neurons. These can be complex additions but are often fairly linear.
Other processing has been found to have much more complex computations that occur when dendrite spikes are involved with multiple different interactions with backward spikes from the axon. One example involves regeneration of pyramidal neurons in the cortex (layer 5). Complex studies show interactions of sodium, calcium and NDMA channels producing multiple dendrite spikes and interacting with inhibitory signals. These studies show that specific complex behaviors in mice are related to non-linear very complex computations in multiple dendrites.
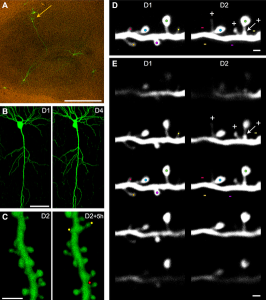
Another set of studies show that dendrites of pyramidal neurons in the cortex layers 2 and 3 respond to visual information with sodium and NMDA spikes that interact with multiple complex non-linear computations. Other studies show simple linear addition for coordination of both eyes. These studies show that there are many different types of integration at work at all times in a small region of the live brain. These include linear additions and other integrations that are not.
Other research for mice spatial orientation with higher order cognition uses many complex dendrite spikes for the integration of information in the hippocampus CA1 pyramidal neurons. Analysis of the mice’s sense of touch uses complex multiple bursts and responds to the integration of many different calcium dendrite spikes. These dendrites can work by themselves or in large complex assemblages.
Neuroplasticity
 Other posts have described the vast amount of different neuroplasticity mechanisms at synapses and now in global myelin production. With so many mechanisms, it is not surprising that research on dendrites has many different findings.
Other posts have described the vast amount of different neuroplasticity mechanisms at synapses and now in global myelin production. With so many mechanisms, it is not surprising that research on dendrites has many different findings.
Some studies of neuroplasticity find the need for dendritic spikes; others show the backward interaction with the axon action potential is prominent. The research shows that neuroplasticity can be related to backward propagation from axon involved timed pulses. When sensory information is being analyzed, NMDA dendrite spikes are necessary for some neuroplasticity. Learning in the motor cortex is correlated with calcium dendrite spikes. What makes this all more complex is that the properties of dendrites change with neuroplasticity (see previous post).
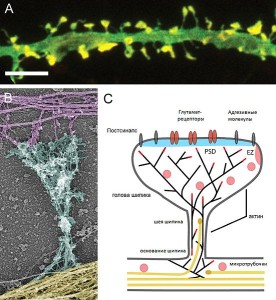
Most research is consistent with the notion that dendrite spikes occur when there are a large number of different inputs to a synapse. At least dozens in very specific small geometrical regions, all occurring at the same millisecond, appear to be necessary in hippocampus and cortex. These inputs have to be in sync in exact locations (see post on Myelin Facilitation of Whole Brain Neuroplasticity). It is, also, found that with neuromodulators and other background activity, they can occur without such intense stimulation.
Some studies have found spikes from inputs that are very far apart in the arbor. The cluster effect occurs with motor learning. But, in the hippocampus there are dispersed signals on layer 5. These occur because the local electrical effects occur independently of all the other inputs. This increases the effects of strong signals to one dendrite. In the clustered type, the local electrical signals merge and interact.

With vast complexity, the clustered and dispersed types of multiple integrated signals can occur in the same neuron as part of the huge arbor. These different types of setups will occur with different situations. In a previous post it was noted that neurons are part of large circuits throughout the brain and neuroplasticity occurs in many different synapse locations with different mechanisms. But, at the next millisecond, the same single neuron is part of a completely different large circuit for a different purpose using different dendrite integrations.
Another research complication is the recent discovery that there are more than a thousand different kinds of neurons. (See post How Many Kinds of Neurons Are There). Each of these has different dendrite characteristics. Also, it is not known if the same type of information goes to all of the dendrites that are connected to one incoming neuron.
How Do Dendrites Make Decisions
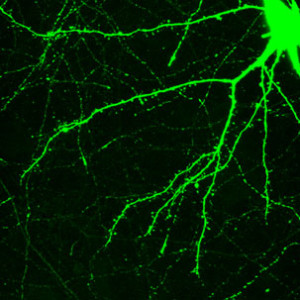
As everything in the brain appears to be vastly complex when studied in detail, the workings of dendrites are just beginning to be described. Previous posts showed that there are fantastic arrays of neuroplasticity mechanisms at different synapses in the same large circuits. These synapses are on particular dendrite spines with their own unique characteristics. Multiple different ion channels make complex variations in electrical functions in dendrites. The spines, the heads and the large dendrites all have different cellular compartments with different characteristics related to the flow of electricity and genetic regulation of proteins. Rapid changes in spines, including sudden appearance of unique geometrical formations are related to neuroplasticity.
The decisions can be from one dendrite, or thousands of dendrites in an arbor of hundreds of thousands. There are now 26 different sizes of synapses magnifying the complexity many times.
All of this complexity responds instantaneously to mental events throughout the brain. No reasonable person can think this is random. Does there not have to be direction from somewhere? Is it not the mind that gives this direction to the diverse molecules, spines, heads, axons and large neuronal circuits?