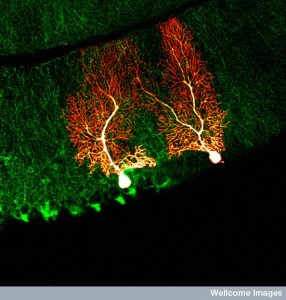
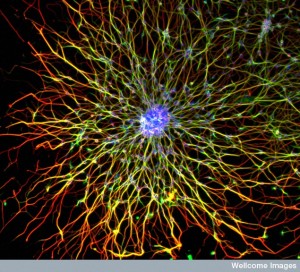
 All mental processes critically depend on extremely active dendrites that receive messages from other neurons and process information. Neuroplasticity in all of its forms is based on altering and modulating the action of dendrites. Unique spacing and shapes of dendrite spines—thin, stubby, mushroom—determine where axons will connect and how the pre and postsynaptic neurons will work together. Each restricts electricity in different ways. Three-dimensional spatial arrangements of synapses are particularly important.
All mental processes critically depend on extremely active dendrites that receive messages from other neurons and process information. Neuroplasticity in all of its forms is based on altering and modulating the action of dendrites. Unique spacing and shapes of dendrite spines—thin, stubby, mushroom—determine where axons will connect and how the pre and postsynaptic neurons will work together. Each restricts electricity in different ways. Three-dimensional spatial arrangements of synapses are particularly important.
A large number of special molecules regulate the flow of information into the dendrite. The dendrite shape is regulated by very elaborate actin scaffolding structures that constantly and rapidly change. And then the dendrite must make meaningful sense of the data—in some way compute the information coming in to send to the next neuron. Rapid determinations are made by the complex dendrite structures—mind interacting with dendrites.
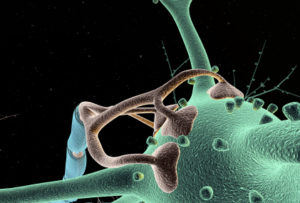
 Dendrites are a unique compartment within the cell in interaction with the cell body and axon. Dendrites are a gate that information flows through from other neurons in the circuit. Dendrites are involved in determining what will occur along the axon. Recently, some of the vast complexity of dendrite function has been discovered.
Dendrites are a unique compartment within the cell in interaction with the cell body and axon. Dendrites are a gate that information flows through from other neurons in the circuit. Dendrites are involved in determining what will occur along the axon. Recently, some of the vast complexity of dendrite function has been discovered.
Dendrites
 Considering relative size, a cell is smaller in comparison with the body, than a human is to Mt. Everest. Likewise, the dendrite and dendrite spine are tiny parts of the neuron. Despite the small size of cells and even smaller dendrites, axons from one neuron can be several feet long from the spinal cord to the toe. This is equivalent in scale to a human walking the wall of China. The dendrite determines much of what will be sent along this vast axon.
Considering relative size, a cell is smaller in comparison with the body, than a human is to Mt. Everest. Likewise, the dendrite and dendrite spine are tiny parts of the neuron. Despite the small size of cells and even smaller dendrites, axons from one neuron can be several feet long from the spinal cord to the toe. This is equivalent in scale to a human walking the wall of China. The dendrite determines much of what will be sent along this vast axon.
Dendrites can have inputs from up to 100,000 different neurons. The analysis of this massive amount of information is done in the dendrite, before determining what information will go forward to the next synapse. Factors that affect decisions include spatial arrangements of the connections on dendrite spines, and the strength and rates of the electrical pulses sent from the neuron. Two neurons might use the same information in different ways.
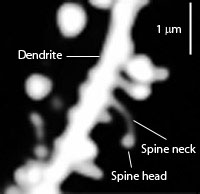
Dendrites are the out pouching areas of the neuron that connect with an axon and form a synapse. The dendritic spine is a protrusion from the dendrite that receives input from an axon. Most spines have a bulbous head and thin neck connecting to the shaft of the dendrite. But, they have many shape variations that undoubtedly have complex different mechanisms.
Dendrite Spines
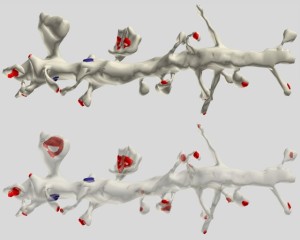
One important factor in neuroplasticity involves changes in the shape dendritic spines. Another is that when one spine is getting a strong signal, others can form nearby. It is not known how they form.
Spine alterations are influenced by multiple factors including BDNF, environment, seasonal change, age and estrogen. Other factors are:
- Stress affects many dendrites at once such as reducing those in the hippocampus and increasing those in the amygdala.
- The particular make up of the postsynaptic density (PSD) is a major factor. This can include over a thousand interlocking proteins in different structures
- Large molecules stick out from either side of the synapse into the space between the pre and post-synaptic cells and shake hands to hold it together—neuroligins on the post-synaptic membrane and b-neurexins from the presynaptic membrane.
Summary of Neuroplasticity
 Previous posts described how learning and memory occur partially through strengthening of particular synapses in wide ranging circuits throughout the brain. These changes occur in many different ways in the varied synapses. They always involve the dendrites. What is surprising is that these alterations in wide ranging circuits across the entire brain can occur instantly in many different ways. A brief list of the many different ways includes:
Previous posts described how learning and memory occur partially through strengthening of particular synapses in wide ranging circuits throughout the brain. These changes occur in many different ways in the varied synapses. They always involve the dendrites. What is surprising is that these alterations in wide ranging circuits across the entire brain can occur instantly in many different ways. A brief list of the many different ways includes:
- Changing myosin motors and actin structures
- New AMPA glutamate receptors
- Changes in complex post synaptic density (1000 interlocking proteins)
- Changes in surface molecules sticking from
- Calcium surge triggers new types of “memory proteins”
- Substitution of new matching neurotransmitters and receptors
- Alteration of signaling cascades from the membrane to the nucleus
- Changes in axon ion channels altering electrical signal
- Change in balance of inhibition and stimulation
- New micro RNA’s alter process
- Mitochondria change strength of the signal
- Climbing fibers in cerebellum with multiple alterations
- NMDA glutamate receptors substitute their subunits
- Transport motors are substituted
- Actin and microtubules direct scaffolding structural changes
- Exosomes send information from astrocytes to neurons including pieces of DNA and proteins
- Complex changes in extracellular matrix molecules that are not in any cell.
- Neuron controlled inflammation pathway
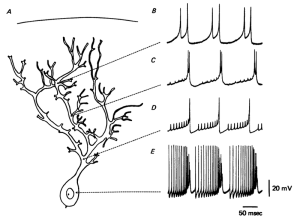
Spine Shapes Determine Electrical Current
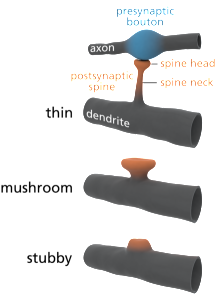
Spines stick out from every part of the dendrite. Each spine has a round head, which is connected to a neck 0.1 μm (0.1 micrometer is a tenth of the size of a small bacterium) that is 0.5 μm long. This makes each spine head a unique molecular and electrical space. Calcium signals and many other different signals determine much about the function and the shapes of the spines.
The shapes of the spine, head and neck restrict the electrical activity in unique ways that make them each function quite differently. Neuroplasticity can operate on one spine or the signals and processes can spread to other spines and the entire dendrite along the shaft into the neuron cell body and the nucleus (several hundred micrometers away). Individual spines compute data and send specific signals to be compared and computed with other signals is special locations in the dendrite.
Constantly Changing Dendrites and Spines
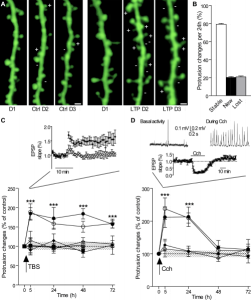
How dendrites and spines multiply is not totally clear. Dendrites behave like an amoeba and produce filopodia in different directions. These protrusions become dendritic spines and are able to attract axons and then connect with them. The mechanism for increased synapses has been thought to involve neurotransmitters producing bulges from the dendrites and axons.
Each spine has a postsynaptic density as well as many receptors. The AMPA glutamate receptors are particularly critical in determining plasticity change. Also, the size of the pre synaptic axon bouton determines activity. The number of AMPA receptors can change, which alters the actions at the spine head including increasing its size. Long-term depression appears to enlarge the spine head and depression shrinks it. The nearby spines do not change size.
The changes of the spine head structure occur first without increased protein synthesis and then synthesis occurs producing many more proteins, which make the alteration more permanent. Calcium signals affecting glutamate NMDA receptors trigger a cascade of enzymes and a great increase in the amount of actin structures. There are, also, events that change the shape in the opposite direction of the neuroplasticity, with LTD associated with spine shrinkage not enlargement. It shows that the processes are extremely complex and not understood. Some show shrinkage in nearby heads that are not even stimulated, which appears as spreading depression.
Dendrite Spine Necks
The neck of the spine electrically shelters the heads by causing resistance to current. This increases the electrical stimulation from the action potentials and creates a potential between the head and the larger shaft of the total dendrite. Longer necks in some types of neurons only affect the volume of the signal that is sent to the neuron’s cell body. Some spines that have very long necks can pick up sub threshold signals. In fact, recent studies show that the necks can increase the signal by 40 times.
It has, also, been demonstrated that necks are regulated independently of other regions of the spine. The stronger the neuronal axon signal, the stronger the neck’s electrical resistance becomes.
Another neuroplastic process makes the neck wider and shorter. Long term potentiation, in general appears to decrease the neck’s electrical resistance. The shorter wider neck allows more resources to travel to the head and therefore can affect particular molecular events occurring in the head. The changes in the neck coordinate with the needs of the unique compartment in the head.
 Proteins from a particular family (septins) are used to make very special shapes in other cells, such as rings, filaments and gauzes during cell division. Septins are active in the base of the neck stopping random membrane proteins from entering the neck membrane. There are, also, special proteins (Ankryns) that connect through the membrane to attach to the actin scaffolding molecules that determine the shape of the spines. A particular Ankyrin-G is active when the head is growing larger.
Proteins from a particular family (septins) are used to make very special shapes in other cells, such as rings, filaments and gauzes during cell division. Septins are active in the base of the neck stopping random membrane proteins from entering the neck membrane. There are, also, special proteins (Ankryns) that connect through the membrane to attach to the actin scaffolding molecules that determine the shape of the spines. A particular Ankyrin-G is active when the head is growing larger.
Post Synaptic Density
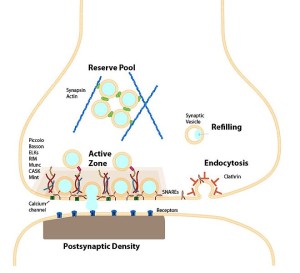
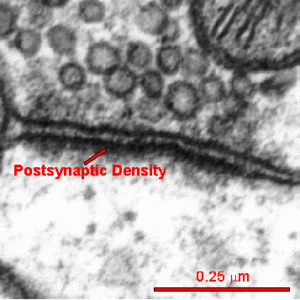 A critical structure in the spine head is the postsynaptic density (PSD), which correlates with the position of active zones (AZ) on the pre synaptic neuron—the region that creates, collects and secretes synaptic vesicles is opposite the area that responds to them. The active zone is the particular part of the axon bouton that prepares for synaptic vesicles to release neurotransmitters. The postsynaptic density can consist of a thousand interlocking large proteins and is different in different neurons, regions and dendrites.
A critical structure in the spine head is the postsynaptic density (PSD), which correlates with the position of active zones (AZ) on the pre synaptic neuron—the region that creates, collects and secretes synaptic vesicles is opposite the area that responds to them. The active zone is the particular part of the axon bouton that prepares for synaptic vesicles to release neurotransmitters. The postsynaptic density can consist of a thousand interlocking large proteins and is different in different neurons, regions and dendrites.
When long-term potentiation neuroplasticity occurs, actin and its adaptor proteins increase the scaffolding, which changes the shape of the spine. At first the PSD doesn’t change; but, hours later the PSD accumulates multiple new proteins and it enlarges. As the PSD grows so does the active zone, stimulated in a retrograde manner from the post to the pre synaptic neuron.
One particularly complex form of PSD is the ribbon release in the retina. They provide very rapid neurotransmitter release needed for vision. The ribbon release sites are in the active zone where neurotransmitter vesicles are docked waiting to be released. They are perpendicular to the membrane and hold a large number of vesicles for rapid neurotransmission.
On the postsynaptic cells, unique structures in the postsynaptic density—PSD95—meet the ribbon release sites. PSD95 clusters are different in each region. PSD95 is a scaffolding structure that brings together many receptors, ion channels and other signaling proteins. It is linked to neuroligin and is very important in neuroplasticity by maintaining the synapse structure during changes in neuroplasticity.
The ribbon release in the pre synaptic neuron rapidly releases and re absorbs multiple vesicles at the same time. Release is triggered by highly regulated graded calcium fluctuations. It is a very accurate rapid process, which is useful for complex sensory systems such as vision and hearing.
New Spines
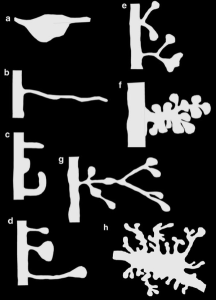
Neuroplastic processes, also, make new spines and other structures such as filopodia. After stimulation this can occur within a minute. The stimulus provides all of the complex machinery involved in making a neck and head. But, existing spines can be eliminated in this same process. Those spines that are least likely to be near neurotransmitters from the active zone are the ones that are eliminated. Over a period of days after the learning that stimulated neuroplasticity, the spines’ positions are remodeled.
The electrical changes in one spine can affect the spines nearby for a period of hours. This can stimulate neuroplastic changes in the nearby spines as well. When multiple spines are stimulated at the same time, then the affect on the nearby spines is in a larger region. The more the stimulation the larger is this affect, such that strong learning and neuroplasticity can entirely remodel the dendrite.
Dendrite Computations
When calcium triggers NMDAR receptors, many different signaling cascades occur that affect future response to glutamate and to the action of the pre synaptic glutamate release. The neurotransmitters stimulate the spines with a brief millisecond increase in calcium lasting a tenth of a second. Repeated signals triggers neuroplasticity to start. Calcium binds to calmodulin triggering a signaling cascade that lasts in the one spine about ten seconds. This cascade triggers other molecules that increase the response to hours and longer.
A very different response involves calcineurin, which spreads the signal to neighboring spines.
Dendrite Structure Depends on Actin Scaffolding
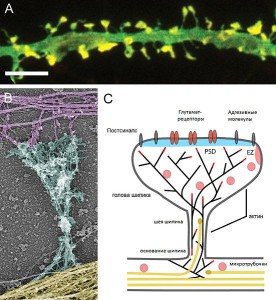
Actin is constantly changing from single molecules (G actin) to long strands (filamentous F actin) that make scaffolding shapes. A single molecule joins the filament at one end (barbed end) and then travels to the other end (pointed) as molecules are added and subtracted constantly. When activated, the actin goes in the opposite direction, or in random directions. Somehow, the flow is altered to orient the direction in line with the neck of the spine toward the large dendrite shaft.
There are a large number of complex proteins that organize the actin movements to make spine shapes. Different molecules have different time frames from milliseconds to hours and have different effects on nearby spines and therefore the region. This adds a large element of complexity in determining the effects of actin on the spine that is stimulated, as well as the entire region. One particular molecule cofilin responds differently when affected by different enzymes. Some cause branching of the actin filaments and other shapes for the spine head.
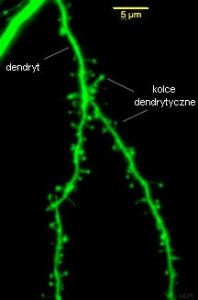
Molecules can easily diffuse and travel from the spine to the larger dendrite. Some of the changes in the actin are local and some travel to the larger shaft. Recent research shows that there are many different compartments in the larger dendrite as well as the spines that effect the larger changes of neuroplasticity. Proteins are mostly made in the dendrite shaft and then moved to the spines. Also, different spatial arrangements of multiple spines form their own compartments.
Special ribosomes exist in the dendrite shafts that use messenger RNAs to make proteins to maintain neuroplastic changes. For example, significant molecules for the postsynaptic density such as PSD-95 are made there and transported to the spine head. Somehow, the local stimulation of spine head stimulates the production of new proteins at a distance. Research shows that messenger RNAs and ribosomes exist in a quite state waiting to be stimulated.
Some Proteins Come from the Nucleus
 Some of the stimulation produces needs for more messenger RNAs from the distant cellular nucleus. The spine is thousands of times smaller than the nucleus, but can stimulate activity rapidly. But, through communication with the nucleus from the spine, special proteins are made in the nucleus and transported to the spine.
Some of the stimulation produces needs for more messenger RNAs from the distant cellular nucleus. The spine is thousands of times smaller than the nucleus, but can stimulate activity rapidly. But, through communication with the nucleus from the spine, special proteins are made in the nucleus and transported to the spine.
It is not clear how such a communication occurs between dendrite and nucleus, but it possibly involves calcium waves through the endoplasmic reticulum to the nucleus. Biochemical signals, also, can travel more than to the local spines. This seems to occur if more than five spines are stimulated. It appears to occur through protein cascades through the cell in a period of up to an hour.
If multiple spines are stimulated in a larger spatial territory, then the signal to the nucleus is more rapid. If all the spines are from one small branch this doesn’t work, but if more branches are stimulated it does. Motor proteins are, also, involved in this communication. Special proteins, like importin, are particular stimuli for the nuclei. The importing cascade and pathway are extremely complex and involve many different pathways.
Once the nucleus makes the new special proteins, they have to be transported back to the very particular spines involved. The changes that occur in the spines with neuroplasticity create a special tag that can grab the proteins as they return from the nucleus. The special conformation of the actin structures can provide a particular way that the proteins return to the correct place. Some nearby spines have these same changes and, also, benefit from the returning proteins.
Vast Complexity in Dendrites
Other posts have described the fantastic complexity of genetic processes and neuroplasticity; another extremely complex process is the transport along the axon.
Here we describe remarkable rapid changes in extremely small dendrite spines that respond to learning and memory. Single spines operate by themselves and in tandem with nearby spines and even far away separate dendrite branches with other spines. The mechanisms are extremely complex including many unusual shapes that have different electrical properties and different spatial relationships that have complex significance.
Computation can occur in one spine, multiple nearby spines, multiple larger branches and over the whole dendrite shaft. This information spreads to the nearby ribosomes and to the nucleus.
Calcium signals activate changes in seconds to a minute. Another calcium signal relays the changes to actin molecules that start changing the shape of the spine heads and necks in hours. Then, the post synaptic density and presynaptic structures remodel in hours.
Where is the direction for all of this? It cannot be in the DNA. The various complex cascades function independently with purpose even even in isolated single spine heads.
No reasonable person can think this is random. Does there not have to be direction from somewhere. Is it not the mind that gives this direction to the molecules, the spines, the heads, the neurons and the circuits?