 Insulin helps control the levels of blood sugar by aiding cells to take in the sugar through the cellular membrane. Simple sugars from the diet can flood the blood and insulin avoids dangerously high levels. It is one of several hormones that are secreted to control sugar levels, which needs to be highly regulated from being to0 high or too low to avoid destructive actions in various places in the body. Normally, excess sugar is stored as glycogen, then fat; then, carbohydrates slowly increase sugar making it easier for the body to handle. Insulin secretion is highly regulated by hormones and cells in the hypothalamus region of the brain.
Insulin helps control the levels of blood sugar by aiding cells to take in the sugar through the cellular membrane. Simple sugars from the diet can flood the blood and insulin avoids dangerously high levels. It is one of several hormones that are secreted to control sugar levels, which needs to be highly regulated from being to0 high or too low to avoid destructive actions in various places in the body. Normally, excess sugar is stored as glycogen, then fat; then, carbohydrates slowly increase sugar making it easier for the body to handle. Insulin secretion is highly regulated by hormones and cells in the hypothalamus region of the brain.
For many years it was thought that insulin didn’t function in the cells of the brain. But, now it is known that there are many different critical aspects of insulin effects in brain cells, including for the developing brain and particularly for specialized cells in the hypothalamus regulating hunger and diet. There are, also, specific insulin receptors in regions of the brain that have different effects that are just being discovered. Recently, it has been found that the greatest number of insulin receptors are, in fact, in the hippocampus, a region of learning and memory. The many ways that insulin can affect cognition are just being discovered. These cognitive effects are not just the dramatic alterations that occur when sugar is too high and too low, but, cognitive effects of insulin itself. It is not yet known how much insulin is transported into the brain at the choroid plexus or whether it is, also, produced in the brain.
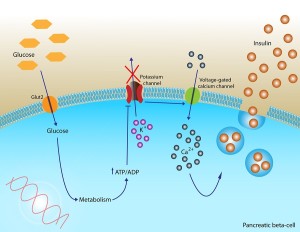
At times, cells become resistant to the effects of insulin. The exact mechanisms of insulin resistance (IR) are not known, but it is caused by high-fat diets, overweight, metabolic syndrome, aging, genes and lack of exercise. Recently, it was discovered that there are different types of insulin resistance. One type develops as part of diabetes and is critical to the overproduction in the pancreas cells, which eventually causes the cells to die. This type is correlated, also, with inflammation. A recent study showed that the type of inflammation from high-fat diets alters microglia to become virulent and eat synapses.
A second type of insulin resistance occurs with aging and is correlated with T regulatory cells that reduce inflammation—the opposite of the type that develops from high-fat diets.
Recently, a third type of cerebral insulin resistance was discovered in the brain. It occurs especially in the hippocampus and is part of the development of cognitive changes of diabetes and dementias.
The relation of insulin resistance and brain disease is very complex. While people with diabetes have more dementia, including Alzheimer’s, diabetes doesn’t seem to increase amyloid plaques and, in fact, there are less plaques in diabetics, although more dementia. Rather, the type of amyloid in diabetes is amylin that precipitates around blood vessels and can cause vascular dementia. Brain insulin resistance (distinct from insulin resistance of diabetes) appears to be unique and it is this type of resistance that is related to brain disease including Alzheimer’s.
This post will discuss recent research attempting to connect insulin resistance in the brain, diabetes, diet, exercise and brain disease. (For details on lifestyle effects on the brain see the previous post Five Secrets of Brain Health.)
Occurrence of Diabetes and Alzheimer’s
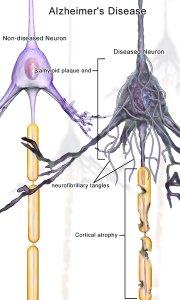
There is 70% more dementia (both Alzheimer’s and dementia caused by blood vessel disease) in those with late-onset diabetes. This means that ten percent of all dementia may come from adult-onset diabetes (called type 2 DM or T2DM). Recent studies show that as the diabetes develops (pre diabetes), especially in those with obesity and lack of exercise, there are already brain changes. All those with metabolic disorders have this risk of more dementia. Although most dementia occurs only in old age, subtle cognitive changes occur in prediabetics and diabetes at all ages. Small cognitive deficits occur in the speed of processing, language, spatial memory and decision-making. In fact, these changes are now called diabetes-associated cognitive decrements. They develop subtly by lowering the percentile of cognitive functioning to the bottom third. They don’t stop life functioning and therefore are, often not noticed. But, the smaller brain volume, decreased connections and lesions, and defective vessels continue to develop as the situation gets worse.
A more severe cognitive state is called mild cognitive impairment (MCI), which produces cognitive changes in the lower tenth percentile of cognitive function and usually occurs in the elderly. Most of these people can still function and do their financial activities. Some have memory loss. Some later develop dementia. The most severe state is dementia with severe cognitive impairments.
MRI studies of those with insulin resistance and adult T2DM without dementia show smaller brain volume (atrophy) in both cells (grey matter) and axon tracks (white matter). This happens especially in temporal and frontal lobes and happens very slowly over years. As people age, many people have smaller brains without diabetes, but those with diabetes and even prediabetes are even smaller. Diabetes affects small blood vessels and there are more small strokes in the brain as well (lacunar infarcts and on MRI hyperintensities of white matter). Measurements of the activity of brain circuits show decreased connectivity of some brain regions. All of these changes are correlated with cognitive decreases.
 Insulin resistance occurs very slowly over many years and is therefore hard to study. It is also very complex in that it is related to inflammation and oxidative stress. Many posts have previously described great complexity in immune function and its relation to the brain. Those who are obese have a variety of different metabolic problems that could independently be related to insulin resistance. It is, also, possible that insulin resistance in brain cells is caused by a different mechanism than those of other cells in the body.
Insulin resistance occurs very slowly over many years and is therefore hard to study. It is also very complex in that it is related to inflammation and oxidative stress. Many posts have previously described great complexity in immune function and its relation to the brain. Those who are obese have a variety of different metabolic problems that could independently be related to insulin resistance. It is, also, possible that insulin resistance in brain cells is caused by a different mechanism than those of other cells in the body.
But, people with Alzheimer’s who do not have diabetes, also, have alterations in the very complex signaling of insulin in the brain. This includes both insulin resistance and, also, specific changes in the signaling cascades triggered by insulin in brain cells.
Diabetes and Alzheimer’s
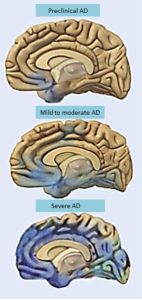 The relationship between diabetes and Alzheimer’s is very complex and not yet clear. For example, people with T2DM have increased risk of Alzheimer’s. However, there is no evidence that the specific causes of T2DM produce the unique pathology of Alzheimer’s.—amyloid plaques and tau fibrillary tangles. But, patients with Alzheimer’s have the cerebral IR. Very surprisingly, T2DM patients have less amyloid plaques and fibrillary tangles. Also, those with general IR do not have more amyloid. Most of the IR have normal sugar metabolism.
The relationship between diabetes and Alzheimer’s is very complex and not yet clear. For example, people with T2DM have increased risk of Alzheimer’s. However, there is no evidence that the specific causes of T2DM produce the unique pathology of Alzheimer’s.—amyloid plaques and tau fibrillary tangles. But, patients with Alzheimer’s have the cerebral IR. Very surprisingly, T2DM patients have less amyloid plaques and fibrillary tangles. Also, those with general IR do not have more amyloid. Most of the IR have normal sugar metabolism.
A different form of amyloid deposition occurs around blood vessels called, amylin, occurs in T2DM. This means that some other process in blood vessels might be related to the dementia of small strokes (vascular dementia). A previous post Does Amyloid Cause Alzheimer’s showed that the amount of amyloid plaques is not consistent in Alzheimer’s. Most people with clinical dementia appear to have multiple causes at the same time.
Insulin Signaling in the Brain
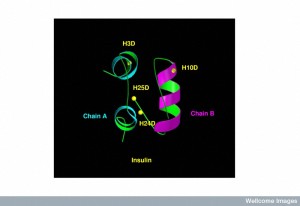 When the receptor for insulin in the cells of the hippocampus is stimulated, it increases cognitive function. The insulin receptor has four different subunits with two ? outside of the cellular membrane and two ? inside the membrane. Many of the cascades of molecules that are stimulated by the insulin receptor are the same in brain cells and other cells. Insulin is secreted by pancreas cells and is transported across the blood brain barrier.
When the receptor for insulin in the cells of the hippocampus is stimulated, it increases cognitive function. The insulin receptor has four different subunits with two ? outside of the cellular membrane and two ? inside the membrane. Many of the cascades of molecules that are stimulated by the insulin receptor are the same in brain cells and other cells. Insulin is secreted by pancreas cells and is transported across the blood brain barrier.
Obesity and metabolic disease can produce adipokines, which are cytokines from fat tissue that can affect brain cells. They are known as immune-modulating agents. However, conflicting data exist about what is termed a cytokine and what is termed a hormone and more research is needed in this area of defining cytokines and hormones. Under the current terminology, adiponectin, leptin, and resistin are not really cytokines since they don’t act on the immune system. These peptides are called adipokines as part of many new hormones that come from fat tissue.
IR eventually leads to increased sugar in the blood, which can, also, affect the brain. Brain effects of insulin alter the amount of food that is taken in and controls metabolism and cognitive function. It is not clear what IR outside of the brain has to do with Alzheimer’s. But IR in the brain is associated with Alzheimer’s. IR in the hippocampus has direct effects on neuroplasticity and therefore memory.
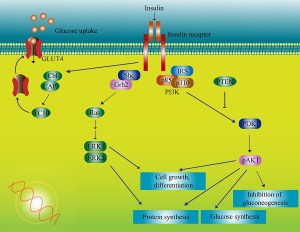 On the ? subunits, a pathway is stimulated that leads to adding energy stimulating phosphorus particles to molecules along a cascade. There are multiple complex pathways that communicate and influence each other. One pathway eventually produces a protein that transports glucose and many transcription factors that trigger genetic networks. There are multiple places in this cascade of twenty steps where impairments can occur in the response to insulin, producing cerebral IR.
On the ? subunits, a pathway is stimulated that leads to adding energy stimulating phosphorus particles to molecules along a cascade. There are multiple complex pathways that communicate and influence each other. One pathway eventually produces a protein that transports glucose and many transcription factors that trigger genetic networks. There are multiple places in this cascade of twenty steps where impairments can occur in the response to insulin, producing cerebral IR.
One animal model in mice uses high-fat diets (HFDs) that approximate Western diets. Some develop obesity and IR and others do not. Some develop cerebral IR.
In mice, T2DM causes impaired learning and memory. Rats on high-fat diet affected complex, but not simple, cognitive tasks, implying frontal lobes and hippocampus. They, also, appeared to have anxiety and depression.
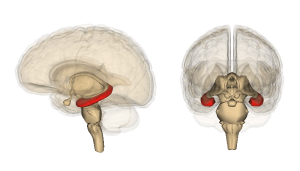
Hippocampus IR demonstrates both altered signaling from the insulin receptor and less transport of insulin into the brain. The alterations in signaling occur at multiple places and these may cause the cognitive loss. Also, alterations occur in the HPA stress pathways of the hypothalamic-pituitary-adrenal axis (see neuroplasticity of stress 1 and 2) with changes in steroid levels affecting IR (increased steroids caused hippocampal IR in mice).
Yet another pathway is neuro inflammation. See the post on how neurons use all four symptoms of inflammation as part of neuroplasticity mechanism. These involve large synapses including neurons, all glia and multiple immune cells at the same time. IL-1?, TNF and IL-6 all are correlated with increased hippocampal IR by different mechanisms. These involve alterations in microglia function, which also occurs in AD. IL-1? appears to be causing neuroplasticity of memory deficits. Outside of the brain, stress steroids from HPA, inflammation and alterations in mitochondria cause IR. There is reason to think this occurs in the hippocampus.
 High fat diets in mice affect the creation of new neurons in the dentate nucleus (neurogenesis). These changes cause spatial learning and memory along with changes in dendrites.
High fat diets in mice affect the creation of new neurons in the dentate nucleus (neurogenesis). These changes cause spatial learning and memory along with changes in dendrites.
Insulin accumulates but can’t be used because of the resistance. This resistance appears in both aging and in obesity. The resistance from obesity appears to be related to an over active inflammatory response. In aging, the opposite occurs. A special type of regulatory T cell (see post on T cells influence on cognition) lowers inflammatory reaction and is related to insulin resistance in aging in mice. When these T cells were not present in the mice, aging insulin resistance improved but not the resistance of obesity.
Depression and Diabetes
 There is evidence that T2DM independently increases depression. When glucose metabolism is controlled people have better mood. In mouse studies, eliminating leptin and leptin receptors, and feeding high fat diet increased signs of behavioral despair. Also, increased IR and decreased insulin receptors did the same. One symptom in the mice is that they eat less sweet treats and appear anxious. As chances of depression increase, managing diabetes becomes more difficult. For example, poor foot health is often a problem with diabetics. Someone with increased chances of depression are less likely to want to solve their foot problems, letting ulcers develop and mismanaging their condition. Someone with less chances of depression are usually more proactive and would be more inclined to visit wholesalediabeticsocks.com to get a pair of diabetic socks and change their diet. Are these effects the same alterations in neuroplasticity already discussed?
There is evidence that T2DM independently increases depression. When glucose metabolism is controlled people have better mood. In mouse studies, eliminating leptin and leptin receptors, and feeding high fat diet increased signs of behavioral despair. Also, increased IR and decreased insulin receptors did the same. One symptom in the mice is that they eat less sweet treats and appear anxious. As chances of depression increase, managing diabetes becomes more difficult. For example, poor foot health is often a problem with diabetics. Someone with increased chances of depression are less likely to want to solve their foot problems, letting ulcers develop and mismanaging their condition. Someone with less chances of depression are usually more proactive and would be more inclined to visit wholesalediabeticsocks.com to get a pair of diabetic socks and change their diet. Are these effects the same alterations in neuroplasticity already discussed?
One study showed that changing the diet from high fat to a better diet helped reduce the symptoms. It is possible that improving diet would help depressed humans as well. There is certainly an interaction of treatment of depression helping people take care of themselves better including controlling their sugar, diet and exercise.
Also, there can be more astrocytes and activated microglia in the hippocampus. The current research points to IR in the hippocampus causing these brain changes in people with T2DM.
Insulin Resistance and Alzheimer’s
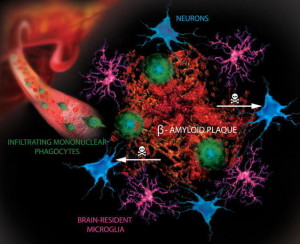 Decrease in insulin receptors activity appears to be part of both T2DM and AD. High fat diets produce abnormal tau (phorphorylated) increases in the hippocampus. Amyloid-? is shown to decrease the insulin receptor’s signals causing increase of IR. There are other complex molecular changes that are similar in the increase of IR in the rest of the body. In Alzheimer’s disease there is, also, decreased function of the insulin receptor in the hippocampus, even without diabetes. These changes correlate with decreases in memory. All of this implies that IR is significant in both diabetes and Alzheimer’s.
Decrease in insulin receptors activity appears to be part of both T2DM and AD. High fat diets produce abnormal tau (phorphorylated) increases in the hippocampus. Amyloid-? is shown to decrease the insulin receptor’s signals causing increase of IR. There are other complex molecular changes that are similar in the increase of IR in the rest of the body. In Alzheimer’s disease there is, also, decreased function of the insulin receptor in the hippocampus, even without diabetes. These changes correlate with decreases in memory. All of this implies that IR is significant in both diabetes and Alzheimer’s.
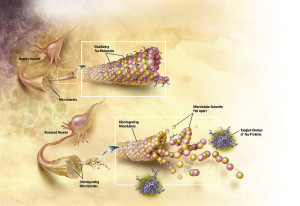 The changes that occur in the cascades of insulin receptor are related to less transport of insulin across the blood brain barrier and decrease of the sensitivity of the receptor. These changes also affect the abnormal tau.
The changes that occur in the cascades of insulin receptor are related to less transport of insulin across the blood brain barrier and decrease of the sensitivity of the receptor. These changes also affect the abnormal tau.
Insulin normally increases cognition. Intranasal insulin increases memory in mice. But, mice fed high fat diets that had cognitive loss didn’t improve. When insulin was placed directly into the mice hippocampus, they improved. But, those fed high fats, did not improve. Therefore, it appears that insulin helps cognition, but not when diabetes changes occur first.
Metformin helps cognition in mice with hippocampus changes from T2DM. Mice fed high fats, did not improved cognition with metformin. Other studies showed metformin increasing neuroplasticity.
Another medication, rosiglitazone, helps metabolic abnormalities of diabetes and it does help the cognitive changes in mice fed high fat diets. Rosiglitazone appears to both help insulin receptors and, also, mitochondria in the brain. This drug stimulates the peroxisome proliferator-activated receptor-? (PPAR ?) related to mitochondrial function. It is called the glitazone receptor, which regulates fatty acid storage and glucose metabolism.

Other factors from the gut are incretin peptides (glucagon-like peptide 1 GLP1 and glucose-dependent insulinotropic polypeptide or GIP), which increase release of insulin. New diabetes medications block the enzyme that breaks down incretins (vildaglipitin and sitagliptin), restores the cognitive deficits and help hippocampus signaling. Another medicine that stimulates incretins (liraglutide) reversed the negative changes in the hippocampus.
These studies imply that it is hippocampal IR, not the peripheral IR, that causes brain problems with diabetes. It is promising that the same lifestyle changes that help avoid diabetes—diet, exercise—reverse these cognitive changes of diabetes. In rats, reduced diets greatly helped mice with these deficits. In mice, exercise, calorie restriction, and gastric bypass reverse the changes.
Nasal Insulin Effects on Alzheimer’s
Intranasal insulin is being looked at to reverse Alzheimer brain changes. It appears to stop abnormal tau. Incretins reverse problems of neuroplasticy. Two of the medications above (liraglutide and lizisenatide) stop the deficits after amyloid is injected into the mouse hippocampus.
Humans, also, have improved cognitive function with intranasal insulin. It is possible that helping the hippocampus insulin receptor can help the brain problems caused by decrease in neuroplasticity from IR in both diabetes and in Alzheimer’s. It is possible that intranasal insulin can help the cognitive loss. Intranasal is used since it goes directly to the brain without the effects that would occur in the rest of the body. A study recently looked at brain glucose, CSF tau and cognition in patents with cognitive loss in mild cognitive impairment and early Alzheimer’s. The intranasal insulin did not affect the tau in the CSF but did affect the glucose metabolism in the brain. But, the larger study is not completed.
The Hippocampus in Type 2 Diabetes and Alzheimer’s
There are factors other than IR that affect neuroplasticity in the hippocampus in people with T2DM. Also, brain IR is only one factor in AD changes in the hippocampus. Changes in mitochondria can lead to hippocampal IR. Both aging and high fat diets create destructive oxidative products. Another different mechanism is genetic alterations of protein glutamate transporters, glucose transporters and ion channels from these oxidative products. Antioxidants help these changes in mice. The endoplasmic reticulum (ER), also, can cause IR (see post on the close relationship of mitochondria and ER). High fat diets increase ER effects. Amyloid plaques itself alter neuroplasticity, unrelated to IR, with multiple different mechanisms.
Hormones and adipokines from fat cells are, also, related to cognitive changes and dementia.
Exercise appears to decrease cognitive decline in the elderly, possibly by these same reversible mechanisms. Exercise, also, helps brain IR, brain sugar metabolism, amyloid plaques and hippocampus atrophy.
Insulin Resistance and Brain Disease
 The vast effects of insulin throughout the body and brain are just being described. It was not known until recently what insulin did in the brain. But, many insulin receptors and sugar transporters have been discovered. These precipitate very complex pathways that are similar to those in the rest of the body, but have different effects.
The vast effects of insulin throughout the body and brain are just being described. It was not known until recently what insulin did in the brain. But, many insulin receptors and sugar transporters have been discovered. These precipitate very complex pathways that are similar to those in the rest of the body, but have different effects.
There are at least three different types of insulin resistance now known—the type associated with type two diabetes with inflammatory changes, the type associated with aging with decrease of inflammation, and the newly discovered cerebral resistance that is related to cognitive loss and dementias. The same triggers occur with both IR of diabetes and cerebral IR—high fat diet and lack of exercise. But, while distinct, both types increase the effects of the other on the brain with increasing cognitive decline. Very recently, another mechanism was found, where high fat diets cause a type of brain inflammation where microglia eat synapses.
The exact mechanism of insulin resistance is not known, but it is clearly very important. This research shows how important lifestyle is for the maintenance of the brain. Please see the post The Five Secrets of Brain Health for more about this.