 The regulation of DNA is fantastically complex with many different layers: changing 3D shapes of the chromatin and loops of DNA; regional differences in nuclear DNA; large numbers of different epigenetic tags on DNA nucleotides and protective protein histone molecules; complex DNA repair mechanisms and alternative messenger RNA splicing; hundreds of thousands of transcription factors; and many different kinds of small and large RNAs that influence every aspect of the process.
The regulation of DNA is fantastically complex with many different layers: changing 3D shapes of the chromatin and loops of DNA; regional differences in nuclear DNA; large numbers of different epigenetic tags on DNA nucleotides and protective protein histone molecules; complex DNA repair mechanisms and alternative messenger RNA splicing; hundreds of thousands of transcription factors; and many different kinds of small and large RNAs that influence every aspect of the process.
Another critical factor of genetic regulation has been recently discovered. Jumping genes make up 50% of all human DNA and the battle between the effects of jumping genes and the cell’s attempts to stop their influence is one of the major drivers of all evolution (see post). Although many different important physiological have been found directly related to jumping genes and viruses, new research now shows that jumping genes directly regulate many vital aspects of brain processes. These extremely complicated mechanisms are now being elucidated. This post will describe vastly complicated jumping genes regulation of the brain.
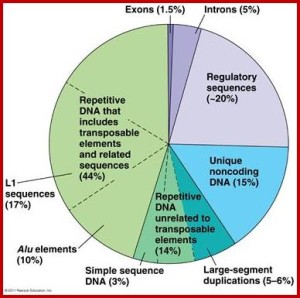 Most are not aware that fifty percent of the DNA in each human cell is in the form of mobile jumping genes—strands of DNA called transposable elements (TE) that have the ability to sew themselves in and out of DNA as well as move to different sections and to place copies in different sections. The mobile strands of DNA in the jumping gene can create new types of proteins, disrupt the entire genetic process and provide new sources of regulation of DNA through many kinds of RNA effects. The jumping gene can provide new epigenetic changes, as well. Previous posts noted that these jumping genes and alternative messenger RNA splicing are especially critical for the human brain and its evolution. A brief summary of this previous post follows.
Most are not aware that fifty percent of the DNA in each human cell is in the form of mobile jumping genes—strands of DNA called transposable elements (TE) that have the ability to sew themselves in and out of DNA as well as move to different sections and to place copies in different sections. The mobile strands of DNA in the jumping gene can create new types of proteins, disrupt the entire genetic process and provide new sources of regulation of DNA through many kinds of RNA effects. The jumping gene can provide new epigenetic changes, as well. Previous posts noted that these jumping genes and alternative messenger RNA splicing are especially critical for the human brain and its evolution. A brief summary of this previous post follows.
From Previous Post
 It is not really possible to distinguish material inserted from viruses, virus-like particles and jumping genes except by their specific content traced to a virus. 8% of human DNA is from retroviruses and has been vital to human evolution. Genes from these viruses include the production of digestive enzymes such as salivary amylase, allowing humans to eat starch. Jumping genes are essential for fetal stem cells and a mother’s immune system; they allowed the production of the human placenta. Jumping genes determine epigenetic changes in the brain and digestive enzymes. In bacteria they are responsible for the spread of antibiotic resistance. Both retroviruses and jumping genes use messenger RNA to make DNA, which is inserted into the human genome.
It is not really possible to distinguish material inserted from viruses, virus-like particles and jumping genes except by their specific content traced to a virus. 8% of human DNA is from retroviruses and has been vital to human evolution. Genes from these viruses include the production of digestive enzymes such as salivary amylase, allowing humans to eat starch. Jumping genes are essential for fetal stem cells and a mother’s immune system; they allowed the production of the human placenta. Jumping genes determine epigenetic changes in the brain and digestive enzymes. In bacteria they are responsible for the spread of antibiotic resistance. Both retroviruses and jumping genes use messenger RNA to make DNA, which is inserted into the human genome.
Bacteria minimize the amount of DNA they use to travel lite and jumping genes don’t proliferated in prokaryotes. Eukaryotes, in contrast, accumulate large amounts of extra jumping genes both copied and altered. One of the major differences between eukaryotes and prokaryotes is the more elaborate epigenetic mechanisms in eukaryotes from this battle with mobile genetic elements.
 An epigenetic immune system in the nucleus battles the jumping genes for control of the cell and control of evolution. In fact, the revolutionary CRISPR, including Crispr-Cas9 laboratory procedure was discovered as part of this process in bacteria. Epigenetic tags of DNA methylation silence retroviruses and jumping genes. Virus DNA produces critical promoters and enhancers that alter human gene function and affect alternative RNA splicing, especially in the human brain.
An epigenetic immune system in the nucleus battles the jumping genes for control of the cell and control of evolution. In fact, the revolutionary CRISPR, including Crispr-Cas9 laboratory procedure was discovered as part of this process in bacteria. Epigenetic tags of DNA methylation silence retroviruses and jumping genes. Virus DNA produces critical promoters and enhancers that alter human gene function and affect alternative RNA splicing, especially in the human brain.
The two opposing forces of mobile elements and epigenetics are very related—the proliferation of mobile DNA particles and the cell’s epigenetic mechanisms to control them. With environmental stress, the genome responds with activation of epigenetic processes. This increases the capacity to withstand this stress using new mechanisms, which can be inherited through epigenetic mechanisms. When the stress subsides, then the increased amount of jumping genes activity also subsides. Recently, more has been discovered about the cell’s response to stress and the regulation by jumping genes that is described in this post.
Jumping Genes in Humans are Unique

There are primarily two types of jumping genes. Type 1 is a strand of DNA that makes a messenger RNA. The jumping gene provides a protein to reverse the normal process, taking the new RNA and making a strand of DNA that is inserted into the gene in a new place. The other Type II is a cut and paste version, where DNA is cut out and then inserted with the help of several enzymes. In human cells, Type 1 is 98% of the jumping genes, representing almost half of the entire human DNA. Technically, Type 1 are called retro transposons.
Mobile jumping genes are found in every eukaryote cell studied thus far. There are three types of retro transposons. These are long interspersed elements or LINEs, short interspersed elements or SINEs and long term repeats LTRs. LTRs are sequences of DNA code that are repeated up to thousands of times. Viruses often use LTRs when they insert their DNA into the human genome. The repeats are created by the special enzymes that past the DNA. LINEs and SINEs make up 30% of the human DNA.
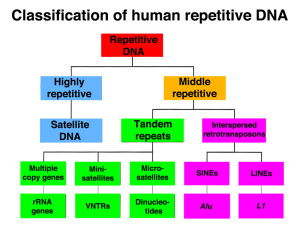 Of the many types of retro transposons, LINEs and SINEs are prominent in animals, which do not have long sections of repeating code. LINEs and SINEs have many important roles in the function of the cell and are important in the brain. They can be transcription factors such as promoters and enhancers that alter the functioning of the rest of the DNA. They can be transcribed along with other genes and affect alternative messenger RNA splicing that was noted to be particularly important in the development of the brain. They can affect the messenger RNA in a wide variety of different ways. They add another large complication to the already fantastically complex genetic regulation with many roles in metabolism, cell division, stem cell differentiation, cellular migration and the response to stress in the brain.
Of the many types of retro transposons, LINEs and SINEs are prominent in animals, which do not have long sections of repeating code. LINEs and SINEs have many important roles in the function of the cell and are important in the brain. They can be transcription factors such as promoters and enhancers that alter the functioning of the rest of the DNA. They can be transcribed along with other genes and affect alternative messenger RNA splicing that was noted to be particularly important in the development of the brain. They can affect the messenger RNA in a wide variety of different ways. They add another large complication to the already fantastically complex genetic regulation with many roles in metabolism, cell division, stem cell differentiation, cellular migration and the response to stress in the brain.
These effects of jumping genes occur in all types of cells and can be important in disease states as well. Current research has found a large number that make non coding RNAs and the effects on only a small number has yet been deciphered. Most remarkably, there is a much greater amount of these mobile jumping genes in the brain.
As a previous post noted, much of evolution is based on the overwhelming positive and negative effects produced by jumping genes and the extremely elaborate defenses against them from the cell.
LINEs and SINEs
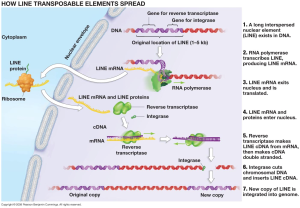 LINEs are several thousand base pair strands produced from DNA into a messenger RNA that produces several critical protein enzymes. These include a protein that binds to the RNA and another than makes a copy of the DNA and inserts it back into the genome. This is done by making two nicks at particular places in the DNA. SINEs, which are 85 to 500 base pairs, don’t make any proteins and instead use those made by LINEs.
LINEs are several thousand base pair strands produced from DNA into a messenger RNA that produces several critical protein enzymes. These include a protein that binds to the RNA and another than makes a copy of the DNA and inserts it back into the genome. This is done by making two nicks at particular places in the DNA. SINEs, which are 85 to 500 base pairs, don’t make any proteins and instead use those made by LINEs.
The placement of LINEs and SINEs are not random. SINEs are in regions where the DNA is most often used to make proteins. LINEs are mostly in other areas. Such a large piece of DNA in a LINE would certainly disrupt any gene. SINEs mix in better with the functioning genes and often develop regulatory functions. Having so many SINEs and LINEs in human cells provide regions of new activity and new problems.
Another surprising recent finding is that proteins provided by LINEs are not just used by SINEs and LINEs, but, also, help other ordinary messenger RNAs to be copied and inserted. These “retro genes” are already useful proteins that are copied in slightly different ways and placed in the genes. These can, also, provide fuel to evolutionary changes along with the jumping genes themselves. They are particularly active in the developing brain’s neurons and in the adult brain (see the post How the Human Brain is Built). Recent studies have found evidence that individual neurons continue to evolve during their long life and become mosaics with many different genetic sequences. In fact, many of the inserted DNA cause problems or do nothing, but the recent research already shows many new functions of the jumping genes.
Regulation of Chromatin

Epigenetic tags are placed on particular base pairs. There are now forty different tags that are known that have important functions in regulating DNA and histones. One of the most important type (or the most well-known) places a methyl group on particular DNA nucleotides. These are very often places in the GC codes (guanine – cytosine). LINEs and SINEs have a very high content of GC codes, therefore are hotspots of epigenetic tags. DNA methyl groups stop transcription in those locations. The CpG sections of the LINES and SINES are methylated and stop the function of the genes nearby.
In fact, the two large categories of chromatin are often defined with a boundary of LINES and SINEs. Heterochromatin is highly condensed in histones mostly at the edge of the nucleus and cannot be used easily because of its bound structure and the many methyl tags on DNA, acetyl tags on histones and other RNAs. Euchromatin is open and loose DNA that can be used. It is mostly in the nucleus center and is very actively producing proteins. LINEs can also block an entire X chromosome through very complex mechanisms.
SINEs can increase gene activity, functioning as enhancers and promoters in the developing brain. This can occur when they are upstream from the gene and have a variety of sites where transcription factors land. It is possible that some SINEs do the opposite and just use up the transcription factors that would otherwise land on the regular binding sites. Both LINEs and SINEs can alter the point that starts the transcription since many have the transcription start sites (TSSs).
Cell’s Response to Stress
 Cellular stress can come from poisoning or infection with viruses. The cell responds to stress by using jumping genes to regulate the number of active ribosomes that make proteins. Regulating ribosomes is a significant way to defend against viruses that take over the cell’s ribosomes to replicate. It is, also, a way to save valuable ribosomes during a crisis until they are needed again.
Cellular stress can come from poisoning or infection with viruses. The cell responds to stress by using jumping genes to regulate the number of active ribosomes that make proteins. Regulating ribosomes is a significant way to defend against viruses that take over the cell’s ribosomes to replicate. It is, also, a way to save valuable ribosomes during a crisis until they are needed again.
When stress occurs, the cell needs to take some of the signals out of commission to prevent triggering cell death and to only use what is necessary during the crisis. It makes “stress granules” to hide this material from general use. These granules gather up other proteins that would be used to produce new proteins. All of this material waits in the granule until the cellular crisis is over so they can be used again normally. When a poison is given, such as arsenic, particular jumping genes form complexes that bind sequestered material into the stress granules. When stress is over, the cell produces more RNAs that are used by the jumping genes and then the sequestered material is released.
This process, also, stops ribosomes from making virus proteins as part of the innate immune system fighting viruses. This is an example of jumping genes helping the cell to fight off random viruses by regulating the use of ribosomes.
The cell generally tries to not allow the function of the RNAs made from SINEs in human organs. But, SINEs respond to stress signals (such as heat shock) and this triggers the SINEs to make a very large amount of RNAs. During this stress many genes are triggered, while ordinary genes are inhibited. In this way the SINEs become an important part of the stress response.
Making RNAs
 Jumping genes have many other complicated genetic effects. SINEs can affect the critical splicing of messenger RNA. One particularly important and very common family of SINEs in humans are called Alu elements. There are many types of Alu elements, which are made of about three hundred base pairs. This strand of DNA was originally named from a bacteria Arthrobacter luteus, which produces an enzyme that cuts DNA in particular places. Alu has been implicated in cancer and other diseases.
Jumping genes have many other complicated genetic effects. SINEs can affect the critical splicing of messenger RNA. One particularly important and very common family of SINEs in humans are called Alu elements. There are many types of Alu elements, which are made of about three hundred base pairs. This strand of DNA was originally named from a bacteria Arthrobacter luteus, which produces an enzyme that cuts DNA in particular places. Alu has been implicated in cancer and other diseases.
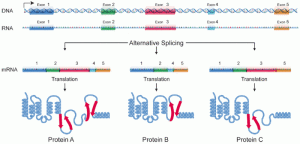
Alu elements have many different effects because of their ability to splice the new jumping genes in many different places. At first they reside in introns (the parts of the RNA that are cut out during messenger RNA splicing). But, they can easily become part of the exon (the part that is used for the protein) and become part of the messenger RNA that will make a protein. A previous post noted that alternative mRNA splicing is most often used in the human brain and is critical for the development of its unique capabilities. Five percent of all alternative splicing in human cells are from Alu sequences. Whenever an Alu piece gets into an exon, it becomes part of the alternative splices. Therefore, it is critical in the development of the unique characteristics of the human brain. A large percentage of the new brain alternative splices (that is, unique proteins) include Alu exons. Somehow, proteins that bind to the RNAs involved with the editing of messenger can associate with the SINE signals and the complex splicing machines.
 Another trick from the Alu is based on the similarity between different Alu versions. Two Alu transcripts can be oriented in different directions and can help form circular non-coding RNAs. Circular RNAs are just now being discovered and many unique properties including regulation of genes. Circular RNA is found in unique regions of the brain and other tissues and in unique stages of development.
Another trick from the Alu is based on the similarity between different Alu versions. Two Alu transcripts can be oriented in different directions and can help form circular non-coding RNAs. Circular RNAs are just now being discovered and many unique properties including regulation of genes. Circular RNA is found in unique regions of the brain and other tissues and in unique stages of development.
A particular sequence of codes is called “polyadenylation” or poly(A) that consists of multiple adenine bases in a row. This poly (A) normally forms a tail at the end of messenger RNAs, which helps as a signal in the normal process of making a protein from the messenger RNA. Also, many of the very long non-coding RNAs that have dramatic effects on the 3D shape of large sections of chromosomes have this sequence at the end called 3’. There are particular code sequences that help create these tails and poly (A) sections in the RNA; these code sequences are called a polyadenylation signal or PAS. LINEs and SINEs are filled with adenine and often produce more of the PAS signals. In particular Alu can create these PAS, alterations in gene functioning are unique in the human species and the human brain.
 The actions of RNAs were described in a previous post (Intelligent Small and Large RNAs) as acting either nearby (cis) or far away and globally (trans). Alu elements in particular have great influence on whether a messenger RNA will be effective based on competition with other elements in the process. Other complex factors are microRNAs made from LINEs and SINEs, which promote more microRNA and which bind as promoters on the messenger RNA. These Alu elements are in 6% of all human messenger RNAs. By using complex mechanisms involving long non-coding RNAs, Alu elements can cause the decay of important messenger RNA mechanisms and influence which proteins are made. In particular, these are influential in neuronal migration.
The actions of RNAs were described in a previous post (Intelligent Small and Large RNAs) as acting either nearby (cis) or far away and globally (trans). Alu elements in particular have great influence on whether a messenger RNA will be effective based on competition with other elements in the process. Other complex factors are microRNAs made from LINEs and SINEs, which promote more microRNA and which bind as promoters on the messenger RNA. These Alu elements are in 6% of all human messenger RNAs. By using complex mechanisms involving long non-coding RNAs, Alu elements can cause the decay of important messenger RNA mechanisms and influence which proteins are made. In particular, these are influential in neuronal migration.
Messenger RNA Locations in Nucleus and Cytoplasm
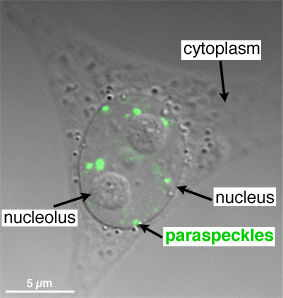 Messenger RNAs are formed in the nucleus from the DNA and then transported outside of the nucleus through the nuclear pores to the vicinity of ribosomes usually in the endoplasmic reticulum to make proteins. However, the unique effects of these SINEs, LINES, microRNAs and Alu elements create a unique compartment called “paraspeckles” that are still in the nucleus. This small compartment near the edge of the nucleus works in conjunction with multiple proteins for RNA functions and long non-coding RNAs. They produce unusual proteins that stay in the nucleus and can influence DNA regulation. Some other Alu elements trigger the proteins to be transported out of the nucleus. Extremely complex processes related to paraspeckles have just been discovered and are not yet fully understood. But, they have definite effects in regulation of DNA.
Messenger RNAs are formed in the nucleus from the DNA and then transported outside of the nucleus through the nuclear pores to the vicinity of ribosomes usually in the endoplasmic reticulum to make proteins. However, the unique effects of these SINEs, LINES, microRNAs and Alu elements create a unique compartment called “paraspeckles” that are still in the nucleus. This small compartment near the edge of the nucleus works in conjunction with multiple proteins for RNA functions and long non-coding RNAs. They produce unusual proteins that stay in the nucleus and can influence DNA regulation. Some other Alu elements trigger the proteins to be transported out of the nucleus. Extremely complex processes related to paraspeckles have just been discovered and are not yet fully understood. But, they have definite effects in regulation of DNA.
The response to stress of Alu elements occurs in the cytoplasm, not the nucleus. Stress granules usually are in the endoplasmic reticulum of the cytoplasm. (Stress granules can sometimes appear in the nucleus.) These are large aggregations of proteins and RNA that are produced when the cell is under stress. It includes many messenger RNAs that did not complete the job of making a protein. The Alu elements attach to enzymes and repress their activity. This can influence a large amount of proteins. They can act nearby or in distant regions where proteins are made. This process is made much more complex during cell replication when there is a breakdown of the separation of nucleus and cytoplasm when the nuclear envelop breaks down. The jumping genes have significant roles in the mitotic process.
Another process increases the use of messenger RNA through long-range mechanisms. Stress, including virus infection and heat shock, increases the amount of Alu RNAs. This, also, occurs when the production of proteins is decreased. This occurs in different ways when other animals are stressed. While the stress is occurring, the Alu stops local factors that inhibit production, thereby increasing production of specific local proteins.
One way cells fight RNA viruses is to alter their RNA by editing adenosine to inosine in the molecule. The inosine is read by the process as a guanosine and therefore the production of sensible proteins is disrupted. Most of these occur in Alu elements.
Fighting Negative Effects of LINEs and SINEs
 While some effects of LINEs and SINEs are positive for cell regulation, many are not and cause disease. Alu elements are the most common insertions in the germ line of humans and in particular make promoters there. Almost a hundred different genetic diseases have been identified from these insertions. Many others occur in the organs, rather than sperms and eggs. Cancers in particular use techniques to remove methylation tags on the LINE and SINE promoters. But, these occur also in brain diseases, including Rhett syndrome and schizophrenia.
While some effects of LINEs and SINEs are positive for cell regulation, many are not and cause disease. Alu elements are the most common insertions in the germ line of humans and in particular make promoters there. Almost a hundred different genetic diseases have been identified from these insertions. Many others occur in the organs, rather than sperms and eggs. Cancers in particular use techniques to remove methylation tags on the LINE and SINE promoters. But, these occur also in brain diseases, including Rhett syndrome and schizophrenia.
One of the major defenses against jumping genes is methylation tags. Recent research finds even more methylation on histones protecting SINEs. Other defense mechanisms include ways to eliminate particular messenger RNAs produced by the jumping genes. Two particular types of small RNAs are active against LINE and SINE RNAs. These are endogenous small interfering RNAs or endo-siRNAs and PIWI-interacting RNAS or piRNAs. (See post on the many kinds of RNAs in the Brain.) There are specific enzyme complexes that cut specific messenger RNA with Alu elements and other jumping genes. Also, autophagy mechanisms gather mobile element RNAs in their lysosomes vesicles.
Jumping Genes Regulation of the Brain
 Recent dramatic findings show that jumping genes are very active in the brain. These SINEs and LINEs are actively altering and regulating neurons and other cells. Some of the changes have been incorporated into day-to-day functions. There is strong evidence that these jumping genes and their effects on alternative functions have been significant in the development of the human brain. This goes along with the evidence that the human brain uses the most alternative messenger RNA splicing. While these findings are still too complex to fully understand, it does appear to be part of the picture that has developed where jumping genes and cellular defense against them are crucial for evolution in general and especially so for the evolution of the human brain.
Recent dramatic findings show that jumping genes are very active in the brain. These SINEs and LINEs are actively altering and regulating neurons and other cells. Some of the changes have been incorporated into day-to-day functions. There is strong evidence that these jumping genes and their effects on alternative functions have been significant in the development of the human brain. This goes along with the evidence that the human brain uses the most alternative messenger RNA splicing. While these findings are still too complex to fully understand, it does appear to be part of the picture that has developed where jumping genes and cellular defense against them are crucial for evolution in general and especially so for the evolution of the human brain.
A previous post How the Human Brain is Built described the unique elaborate genetic networks and clusters determine the fantastically complex human brain. These findings about jumping genes up the ante considerably in understanding the complexity of genetic regulation in the brain.