 The largest number of brain molecules are lipids (fats). Unique regulation of brain lipids is complex and contributes to many diseases. Surprisingly, it has been found that membrane lipids direct proteins and proteins direct lipids.
The largest number of brain molecules are lipids (fats). Unique regulation of brain lipids is complex and contributes to many diseases. Surprisingly, it has been found that membrane lipids direct proteins and proteins direct lipids.
Previous posts have discussed the importance of lipids in communication between brain cells using vesicles made with fatty membranes. The rapid complex process where lipid covered vesicles transmit neuro transmitters at synapses uses 80% of all of the brain’s energy. Secretory pathways in neurons that form fatty membranes for vesicles are extremely complex. Large complexes of many interlocking proteins are involved assembling machinery for lipid-based processes. The unusual collaboration of unique lipids and proteins in membranes are just being discovered.
Lipids in the Brain
Lipids in the brain are unusual in that they are insoluble, but at the same time form complex structures in water solution. Brain has more varied lipids than any other organ and elaborate lipids are correlated with higher cognitive ability in primates. Lipids in brains vary at different ages, in different brain regions and with stress and trauma. Changes in lipids are associated with many psychiatric and neurological diseases.
 Vital pre synaptic terminals engage in very rapid and complex remodeling of fatty membranes. Please see the previous post on Mysterious Pre Synaptic Vesicles for a discussion of the complexity of this process. It is still not completely understood.
Vital pre synaptic terminals engage in very rapid and complex remodeling of fatty membranes. Please see the previous post on Mysterious Pre Synaptic Vesicles for a discussion of the complexity of this process. It is still not completely understood.
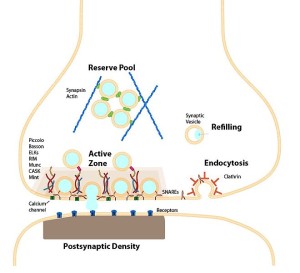
Membranes are dramatically and very rapidly altered to provide constant release of vesicles filled with neurotransmitter molecules. Vesicles somehow are released from the membrane without making holes and then are recycled and re used milliseconds later. Protein structures build scaffolding for this release in the form of clathrin and SNARE machinery discussed in previous posts.
Chicken and Egg
 New research shows lipids regulating all of the complex membrane processes. But, proteins help to rapidly produce unique lipids for each situation. They both direct each other. Another well known chicken and egg conundrum is DNA making proteins and proteins regulating DNA. How do these interactions develop? At synapses, lipids control protein activity and proteins control what types of lipids are produced.
New research shows lipids regulating all of the complex membrane processes. But, proteins help to rapidly produce unique lipids for each situation. They both direct each other. Another well known chicken and egg conundrum is DNA making proteins and proteins regulating DNA. How do these interactions develop? At synapses, lipids control protein activity and proteins control what types of lipids are produced.
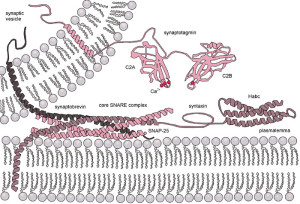
Lipids do more than provide a barrier. They are uniquely interactive with proteins in all processes. Terminals are extremely efficient at remodeling membranes. Vesicles fuse with membranes in 1 millisecond and can reproduce this process 100 times per second or more.
Vesicles are released from special active zones with elaborate SNARE machinery including many large interacting proteins. Active zones include a large amount of interconnected proteins that prepare and dock vesicles and help to release them. Vesicles are also taken back in to membrane of the synapse. Somehow, membranes are rapidly brought back to the normal state to prepare for the next alteration. If membranes did not go back to the normal state, they would rapidly deteriorate with proteins and lipids in the wrong places.
Special Lipids in Membranes
 Synapses contain cholesterol and poly unsaturated fatty acids (PUFA – see post on Polyunsaturated Fatty Acid Signaling in the Brain). Vesicle mechanisms need other particular lipids including phosphatidylinositol phosphates and phosphatidylserin (PtdinsPs and PtdSer). Many different enzymes metabolize lipids in synapses that alter their structures.
Synapses contain cholesterol and poly unsaturated fatty acids (PUFA – see post on Polyunsaturated Fatty Acid Signaling in the Brain). Vesicle mechanisms need other particular lipids including phosphatidylinositol phosphates and phosphatidylserin (PtdinsPs and PtdSer). Many different enzymes metabolize lipids in synapses that alter their structures.
PtdinsPs are negatively charged lipids facing the inside of the cell. They are critical in multiple steps of neurotransmission process. Seven different PtdinsPs are used in various phases based on where phosphates are placed on the molecule and how many placements there are. Although there is only a small concentration of each lipid molecule in membranes, many of the proteins in the synaptic machinery have unique interactions with each type. These specific lipid molecules define unique compartments in the membrane.
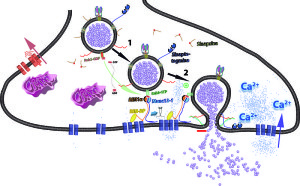
Special interactions between particular lipid versions and unique proteins are vital where vesicles are released in synapses. Collaborations are necessary for neurotransmitter release. Lipid molecules regulate how pre synaptic vesicles are released by controlling calcium. It has been known for some time that when action potentials arrive at axon terminals, calcium is increased and this somehow triggers release of pre synaptic vesicles filled with neurotransmitters. But, only recently has the direction by lipid molecules been discovered.
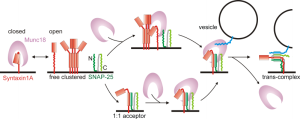
Particular neurons that release hormones have special lipids placed in unique places at terminal membranes. They are close to the SNARE machinery and many vesicles docked and ready for release. Proteins (Syntaxin1A) form dense clusters of vesicles ready to be released and these bind to special lipid molecules. Molecular connections occur between vesicles, lipids and proteins that run through the entire membrane, ready to release a vesicle. Each Syntaxin1A binds to five lipid molecules (Ptdins(4,5)P2). Interactions of protein and lipid form special clusters. In studies where these lipids are metabolized, transmission doesn’t occur.
Lipids with negative charge also bind to other proteins in active zones at the same time. These are related to preparing vesicles and their docking sites. Docking sites and vesicles bind together because specific domain structures in active zones are positively charged. Lipids connect docking structures to membrane proteins. Other more abundant negatively charged lipids join in the mechanism. Because membranes are fluid, these many mechanisms are necessary to stabilize the entire operation. Together they release the vesicle.
Lipids Regulate Proteins
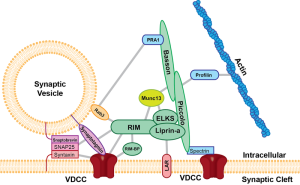
The SNARE machinery is very elaborate with many large interacting molecules. These have regions with positive charge. Positively charged regions bind with two negatively charged special lipids. Lipids have the ability to shield other positive charged proteins as they approach the SNARE complex.
Other lipids help in this directed process to bind particularly with SNARE. In fact, enzymes cut different lipids (sphingolipids) processing positively charged lipids to help this complex process of connecting to original negative lipids. Many different lipids control electrostatic charges from all proteins present in complex terminals. In this way, they organize so that the critical negative lipid meets a precise positive domain in the active zone.
 PtdinsPs also coordinate ion channels that create electric charge in neuronal membranes. One unique lipid in particular controls the shape of the ion channel, so that only particular combinations of proteins and this particular lipid can allow ion exchange to occur. These have been shown to be critical in potassium channels that maintain axon resting electrical charge. Special lipids again interact strongly with proteins that lie across membranes. Research shows that only with this interaction are channels placed properly in membranes.
PtdinsPs also coordinate ion channels that create electric charge in neuronal membranes. One unique lipid in particular controls the shape of the ion channel, so that only particular combinations of proteins and this particular lipid can allow ion exchange to occur. These have been shown to be critical in potassium channels that maintain axon resting electrical charge. Special lipids again interact strongly with proteins that lie across membranes. Research shows that only with this interaction are channels placed properly in membranes.
As electric potential changes during an action potential, lipids help change shapes of channels for this process. They also help ensure that when not needed, channels are inactive. When action potentials reach the terminal, these same lipids are involved in activation of calcium channels. Calcium is positively charged and neutralize negative lipid charge as part of the mechanism.
Local small regions of special lipids keep correct proteins at sites needed to release pre synaptic vesicles. Lipids provide exact sites where vesicles merge with membranes and are released. They do the same when vesicles are picked up into membranes to be recycled. Lipids also allow placement of vesicles before release and the release mechanism. They regulate ion channels that create electric charge in the axon.
After Release
 Just after release of a vesicle, lipids and proteins are grabbed back into the cell in a very short time. This occurs near the edge of an active zone that is preparing for more vesicles to be released. It is much slower to build protein clathrin scaffold structures for the vesicle returning to the cell. They can also come in as a group in another region (bulk endocytosis).
Just after release of a vesicle, lipids and proteins are grabbed back into the cell in a very short time. This occurs near the edge of an active zone that is preparing for more vesicles to be released. It is much slower to build protein clathrin scaffold structures for the vesicle returning to the cell. They can also come in as a group in another region (bulk endocytosis).
The same negatively charged lipids are necessary for the clathrin process and possibly in other mechanisms. In the same way, negatively charged lipids bind to all necessary proteins in complex ways. Special lipids are necessary for the sites that bring vesicle back in at the periphery of the active zone.
Making Lipids
 Proteins that are critical in all phases recognize only one or a few of these specific lipid molecule variations. Specific lipids are manufactured for particular jobs and define different sites by their ability to bring proteins together. Most is known about how this occurs in the process of vesicle release in the different compartments. Unique lipids define different sub regions.
Proteins that are critical in all phases recognize only one or a few of these specific lipid molecule variations. Specific lipids are manufactured for particular jobs and define different sites by their ability to bring proteins together. Most is known about how this occurs in the process of vesicle release in the different compartments. Unique lipids define different sub regions.
Even more complex is that unique lipids are altered by particular enzymes (kinases take off phosphates and phosphatases add phosphates) to make different variations. Enzymes alter structures of local machinery by changing lipids that attract different proteins to a region. Many enzymatic changes make the situation much more complex and hard to research. To make this even more complex, several of the enzymes can make multiple different versions of unique lipids.
In the process of bringing back vesicles using protein clathrin coats, enzymes produce many specific lipids. First, one form of lipid is used. Then when other proteins are needed, enzymes alter each lipid to attract other proteins. There appears to be a back and forth interaction between the lipid and protein molecules that regulates the entire process—lipid regulating proteins and protein enzymes regulating lipids.
Diseases From Altered Enzymes
 If these enzymes are altered by mutations, diseases occur in brain and elsewhere. Gene mutations can affect either kinases or phosphatases. Another enzyme that helps originally produce lipids can have mutations as well. This later mutation affects spinal cord anterior horn cells causing a debilitating syndrome with joint contraction, loss of muscle and nerves that affect breathing. There are other versions of this severe deadly syndrome.
If these enzymes are altered by mutations, diseases occur in brain and elsewhere. Gene mutations can affect either kinases or phosphatases. Another enzyme that helps originally produce lipids can have mutations as well. This later mutation affects spinal cord anterior horn cells causing a debilitating syndrome with joint contraction, loss of muscle and nerves that affect breathing. There are other versions of this severe deadly syndrome.
Many diseases are caused by various different mechanisms. Alterations in lipid metabolism causes Friedrich’s ataxia, a more common disease with progressive loss of nerves connecting cerebellum and spinal cord. Another mutation can cause a very early version of Parkinson’s disease and epilepsy. This latter mutation stops activity of Ptdins3Ps and PtdinsPs involved in synaptic vesicles and in lysosomes (vital vesicles that clean cellular debris and microbes). These lipids are also part of Down’s syndrome and the Alzheimer’s version of Down’s syndrome. The risk factor for the more common Alzheimer’s involves APOE4, which lowers these lipids.
Membrane Characteristics
 Shapes and flexibility of membranes are crucial to their functions. These same unique lipids contribute to thickness of membranes and its ability to form curves. The amount of particular lipids with cone shapes determines whether membranes can effectively form a curve, which is necessary for all round vesicles and for fusion with membranes and vesicles. Spherical vesicles are the most difficult of all membrane curves to keep stable.
Shapes and flexibility of membranes are crucial to their functions. These same unique lipids contribute to thickness of membranes and its ability to form curves. The amount of particular lipids with cone shapes determines whether membranes can effectively form a curve, which is necessary for all round vesicles and for fusion with membranes and vesicles. Spherical vesicles are the most difficult of all membrane curves to keep stable.
Local amounts of these special lipids allow for correct bending. Sometimes, specific lipids are produced and replace others for rapid bending needs. The amount of fatty acids and cholesterol affects flexibility. Placement of these particular lipids at the exact timing of a bend is vital to the entire vesicle process.
Cone Shaped Lipids
 Cone shaped lipids are necessary for curves and bending. They are part of the fusion processes and alter amounts of energy needed to make curves. Special lipids promote positive and negative curves. Ceremide, phosphatidic acid (PA), diacylglycerol (DAG) and lysophospholipids cause negative curvature in sphere. They are found near SNARE machinery in the active zones.
Cone shaped lipids are necessary for curves and bending. They are part of the fusion processes and alter amounts of energy needed to make curves. Special lipids promote positive and negative curves. Ceremide, phosphatidic acid (PA), diacylglycerol (DAG) and lysophospholipids cause negative curvature in sphere. They are found near SNARE machinery in the active zones.
Cone shaped lipids on the inner half of membranes (cytoplasmic leaflet) cause fusion with vesicles. Inverse cones on outer leaflets can do the same. Therefore, there are many options for membranes that are fusing with vesicles for exocytosis and endocytosis. Calcium can trigger these particular lipids with an enzyme called lipase. Lipases make the appropriate lipids for neurotransmission.
Enzyme remodeling of lipids is vital for all aspects of synapse activity. Alterations in enzymes produce many different severe neurodegenerative diseases. Different diseases have various mutations of a gene for phospholipase. How these mechanisms operate has not been figured out yet. Very specific lipid functions are vital to function of the brain. Many other types of enzymes related to general lipid metabolism also produce specific neurological diseases.
Lipids have Water Loving Sections
![]() Many different kinds of fatty acids vary in length and chemical characteristics. They have many different relations with water solutions. Synapses have a large number of different PUFAs (polyunsaturated fatty acids – see post on PUFA signaling). PUFAs help synapse machinery because they strengthen regions of membranes that stay away from interactions with water. They are also more flexible. Mechanisms that alter membrane shapes to produce vesicle fusions are greatly helped by PUFAs. They also avoid any chance of very curved membranes to allow interactions with water and possible membrane breaks. The curve of a vesicle is the sharpest curve of all organelles.
Many different kinds of fatty acids vary in length and chemical characteristics. They have many different relations with water solutions. Synapses have a large number of different PUFAs (polyunsaturated fatty acids – see post on PUFA signaling). PUFAs help synapse machinery because they strengthen regions of membranes that stay away from interactions with water. They are also more flexible. Mechanisms that alter membrane shapes to produce vesicle fusions are greatly helped by PUFAs. They also avoid any chance of very curved membranes to allow interactions with water and possible membrane breaks. The curve of a vesicle is the sharpest curve of all organelles.
Large amounts of cholesterol in synapse membranes affect lipid structures and flexibility. Cholesterol is necessary for synaptic vesicles. Cholesterol can move from inner to outer leaflets of membranes relaxing stress by filling in space between lipid molecules in very curved membrane. Cholesterol also defines specific compartments of membranes and SNARE machinery.
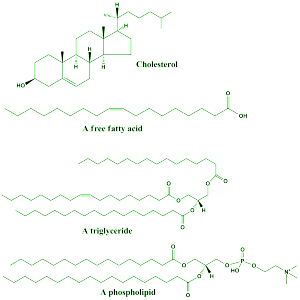
Structures that make membranes more flexible and strong control protein shapes in membranes and their activity. Flexible PUFAs are necessary for functional protein shape changes such as channels. Vision requires PUFAs in rats and non-human primates. It helps in two ways, increasing membrane flexibility and increases lateral diffusion of elements for signaling.
Proteins sitting in membranes are affected by rigidity and thickness. Gamma secretase enzymes cut APP molecules to make amyloid plaques in Alzheimer’s. Cholesterol modulates effects of secretase by keeping it in certain positions, cutting APP in thicker deeper membranes. Deeper membrane cuts are more likely to make plaques. When certain fatty acids are in membranes, less toxic types are produced. Different membrane thicknesses from special lipids affect properties related to interactions with water. These changes in relation to water alter how cuts are made. This is not fully understood, but there are many other lipids that interact in Alzheimer’s.
Lipids and Proteins Cooperate in Membranes
All of these membrane characteristics occur at the same time, such as flexibility, positive and negative charges, and thickness. Proteins influenced by lipids are all affecting lipid structures as well. Proteins are also critical in stabilizing membrane curves. Proteins sense differences in lipid structures. Sections of proteins have different interactions with lipid and water. When membranes are highly curved, proteins can stabilize by inserting strands into lipids.
Negatively charged PtdinsPs attract special proteins to unique regions in the membrane structure. These create stresses in various places and help form vesicles in multiple different parts of the process. They help make pits, control their size, form vesicle structures and help membranes break away from the membrane after fusion. These proteins change characteristics and can be soluble and can then change and be part of lipid non-soluble membranes. Multiple different strands and sub domains on proteins can interact with many different situations. Some even become links to specific cargoes.
Lipid protein interactions are highly complex and alter membranes and also attract necessary cargoes and machinery to make more vesicles. α-synuclein is a prominent synaptic protein that also has domains that bind lipids in membranes. Abnormal α-synuclein causes Parkinson’s disease. It becomes insoluble and produces Lewy bodies. What α-synuclein does in normal membranes is not clear, but it is one of the important proteins for presynaptic membrane remodeling. Lipids control different shapes of α-synuclein molecules. Abnormal types form beta sheets.
Membrane Lipids Direct Proteins and Proteins Direct Lipids
Interaction of lipids and proteins are very complex and are just being researched. Presynaptic release of neurotransmitters is extremely efficient and rapid. Every step is very complex including altering the membrane to start the process, fusion of vesicles with membrane, sorting material afterward and fission or taking the vesicle out of membranes.
To accomplish each step, very specific lipids create sub domains in the membranes attracting and working closely with many kinds proteins. Unique lipids determine the various complex structures in membranes needed for all aspects of vesicle release. This includes production and docking of a large amounts of vesicle ready to be used. Lipids direct all the many proteins involved all of these phases of synaptic transmission.
But, proteins produce many variations of the lipids for each use with many different enzymes. In one case, lipids and proteins regulate enzymes that metabolize lipids changing the membrane composition. In many other cases enzymes determine how the special lipids are produced.
In one of the notable chicken and egg phenomenon in biology (such as DNA making protein and protein regulating DNA), membrane lipids regulate proteins and proteins regulate and modify lipids. It is very hard to research lipids in a membrane, so this story is just beginning.