 Until quite recently it was thought that only 2% of human DNA making proteins was important. The rest was called “junk.” Junk DNA was thought to accumulate through random processes with no physiological consequences. After the genome project did not find enough “mutations” to explain more illnesses or to explain evolutionary change, 98% of DNA that was left took on new meaning. Ten years after the genome project, ENCODE (research involving over 100 institutions world wide for the encyclopedia of DNA elements) found that a great percentage of the 98% of DNA was significant and operated by forming small and large RNAs that had vital physiological functions. Debate about regulatory DNA now places vital DNA between 20% and 40%. Even at the smaller figure, regulatory DNA is ten times larger than “genes”. It is, also, now clear that 50% of the total DNA consist of jumping genes that have critical importance in evolution (see post on jumping genes).
Until quite recently it was thought that only 2% of human DNA making proteins was important. The rest was called “junk.” Junk DNA was thought to accumulate through random processes with no physiological consequences. After the genome project did not find enough “mutations” to explain more illnesses or to explain evolutionary change, 98% of DNA that was left took on new meaning. Ten years after the genome project, ENCODE (research involving over 100 institutions world wide for the encyclopedia of DNA elements) found that a great percentage of the 98% of DNA was significant and operated by forming small and large RNAs that had vital physiological functions. Debate about regulatory DNA now places vital DNA between 20% and 40%. Even at the smaller figure, regulatory DNA is ten times larger than “genes”. It is, also, now clear that 50% of the total DNA consist of jumping genes that have critical importance in evolution (see post on jumping genes).
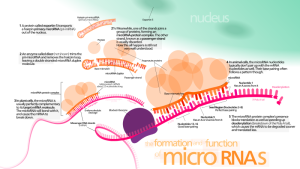
Thousands of small RNAs have now been found to be vital in all aspects of physiology and genetic regulation. Many large RNAs have dramatic global effects on structure and function of the entire chromatin. Recently it has been found that in psychiatric illness many have altered microRNAs in the brain and in the blood. This post will discuss current findings about psychiatric illness and microRNA. Experiments with animals can show altered behavior with specific microRNAs. These might one day be part of the diagnosis and treatment of brain disease and psychiatric illness.
A previous post described difficulties in characterizing the genetics of psychiatric illness despite strong correlations. Few specific mutations have been correlated exactly with mechanisms of psychiatric illness. Many environmental factors appear to play some role in psychiatric illness including physical and mental stress in early life. Epigenetic mechanisms might also be important, such as tags on DNA and histones, alterations of proteins and RNAs. Small RNAs have been found recently to be a prominent way that alterations in gene expression occur in many areas, notably the brain.
MicroRNA
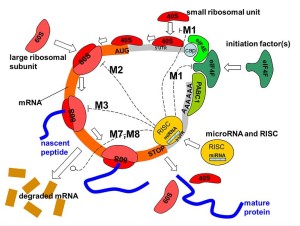
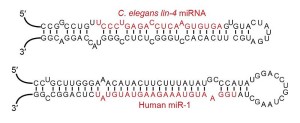
Each microRNA can alter hundreds of different genes (MicroRNA is usually designated as miRNA – I am using microRNA for ease of reading). Studies show that they are involved in regulation of half of all genes. MicroRNAs have approximately 22 nucleotides. They become part of a complex to silence (alter) existing messenger RNAs about to make proteins. MicroRNA can affect codes in several ways. They can silence a particular strand of messenger RNA. They can disrupt the process. They can block genes. They can slightly alter the results, as often happens in adult neurons. They alter neuronal networks by tuning gene expression in particular ways.
Most research has been with microRNAs in the fetus, during neuroplasticity and in brain disease. But, they also can affect behavior and mood. Current theories of psychiatric illness are looking at altered gene expression that changes brain structures and their activity. Studies include animals and humans, as well as studies of cells. Because of the great complexity involved, multiple approaches are often used at the same time.
Making a MicroRNA
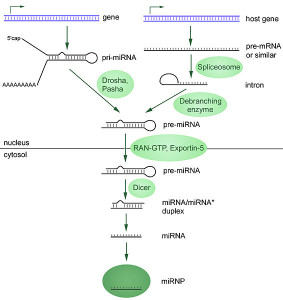
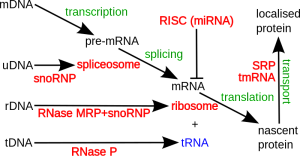
Making a microRNA is surprisingly complex. The large RNA polymerase II and III enzymes transcribe microRNAs from DNA. First they make “primary transcripts” from specific genes or from other genes where parts of the DNA (introns) are edited. Either a pri form (primary) as individual microRNAs or clusters are made.
In the first steps of this process, RNA makes a dramatic hairpin curve. Next, editing is done by complex enzymes (ribonucleases) that cut them further, breaking the hairpin turn. Another molecules serves as a ruler to determine the exact places for cuts. Then it is further cut to smaller size of 70 to 110 nucleotides, where it is called a “precursor microRNA”. This is sent out of the nucleus to the cytoplasm where it is cut again by a different enzyme (called DICER) making a double microRNA around 22 nucleotides long.
Then, it is sent to a large complex called RNA induced silencing complex or RISC that helps it silence a gene by placing it in the proper place. One piece of the duplex is complementary to the messenger RNA. The other strand is broken down. RISC takes it to messenger RNA to affect how it is used. Before the final stage it is called mir, but once it is made the microRNA is called miR. An example is miR-35.
Alterations in any of these steps—primary microRNA (pri-miRNA), precursors or pre-miRNAs, mature microRNAs or other factors can change how much microRNA is made. Changes in gene function through tags and different structure can increase or decrease the amounts and the effects. It is a very complex process.
Research In Humans
 See the post on genetics of psychiatric illness for details on the use of specific association studies. Studies on microRNAs have shown changes in chromosomes with codes that are deleted, added and duplicated. These are called copy number variations or CNVs. CNVs are looked for in regions where specific microRNAs have been found in psychiatric illness. Specifically research has looked at sequences in genes that match the microRNA sequences. These are called seeds and seed matches.
See the post on genetics of psychiatric illness for details on the use of specific association studies. Studies on microRNAs have shown changes in chromosomes with codes that are deleted, added and duplicated. These are called copy number variations or CNVs. CNVs are looked for in regions where specific microRNAs have been found in psychiatric illness. Specifically research has looked at sequences in genes that match the microRNA sequences. These are called seeds and seed matches.
One possible important microRNA is called miR-137. Genome wide association study or GWAS of 40,000 people found that miR-137 somehow related to risk for schizophrenia. In this study risk was also associated with a particular version of a gene T rather than G. Normal people had less of miR-137 than schizophrenics. Before these studies associated with psychiatric illness, miR-137 was thought to be related to making new neurons. A large follow up study found the same result. Those with two risk genes in both complementary DNAs were more likely to have schizophrenia and greater changes in brain structure (larger ventricles, less axons and small hippocampus).
 Another study showed miR-137 is associated with fMRI changes in the medial frontal gyrus and is correlated with decreased cognitive abilities. With emotional tasks, other regions were altered such as frontal amygdala and DLPFC (dorso lateral pre frontal cortex). Other studies showed that other genes that are targeted by miR-137 might also be involved in schizophrenia. In fact, four of the places found in the GWAS were targets of this microRNA.
Another study showed miR-137 is associated with fMRI changes in the medial frontal gyrus and is correlated with decreased cognitive abilities. With emotional tasks, other regions were altered such as frontal amygdala and DLPFC (dorso lateral pre frontal cortex). Other studies showed that other genes that are targeted by miR-137 might also be involved in schizophrenia. In fact, four of the places found in the GWAS were targets of this microRNA.
Looking at patient’s brains who have died with psychiatric illness found some particular microRNAs. Although these microRNAs are found after death in a given region, it doesn’t prove it is important in causing problems in life. One study related to major depression found down regulation of miR-1202 in prefrontal cortex. This was found in subsequent studies as well. The finding was worse when there was also anxiety and treatment with antidepressants. This microRNA appears to be somehow related to a glutamate receptor (GRM4).
Another study of miR-135 was related to serotonin in the raphe nucleus in patients who had killed themselves. This microRNA appears to be related to the serotonin transporter (SERT SLC6A4) and receptor 5HT1A.
In Blood
 MicroRNAs are found in blood and blood cells. They are especially in membrane vesicles, exosomes and bound to proteins. It is not clear how they end up in blood. They may come from cells that died or are secreted actively as signals. They may act as hormones with distant effects (see post on vesicle signals). There is evidence of communication of cells using microRNAs. They may affect nearby brain regions. Previous posts have described microRNAs as part of the vesicle and nanotube signaling between neurons, glia and immune cells.
MicroRNAs are found in blood and blood cells. They are especially in membrane vesicles, exosomes and bound to proteins. It is not clear how they end up in blood. They may come from cells that died or are secreted actively as signals. They may act as hormones with distant effects (see post on vesicle signals). There is evidence of communication of cells using microRNAs. They may affect nearby brain regions. Previous posts have described microRNAs as part of the vesicle and nanotube signaling between neurons, glia and immune cells.
MicroRNAs in blood have been used as markers for cancer and diabetes. There is more evidence that they are relevant in psychiatric disorders. Study of miR-1202 in depression has the most research. Those patients that responded to antidepressant treatment had different levels of the microRNA. miR-1202 levels are lower at first in those who eventually respond to treatment, but then become greater when the medication works. miR-135a levels were, also, lower with depression and increased when the patients responded to treatment. These were not big studies and need to be repeated. Other studies used miR-16 might also be correlated. The question is whether these microRNAs are involved in the mechanism of depression and its cure.
Another approach is to take blood cells such as lymphocytes from psychiatric patients and study their lineage. But, generation of cell lines may alter the epigenetic mechanism, so this research is just beginning.
In Mice
In animals, microRNA patterns for each brain region can be described. This can be studied in relation to behavioral changes. Some of these studies involve genetic models where genes are altered in a line of mice. Another type of study uses genetically normal mice who are stressed in different ways and treated with medications. These studies might be relevant in humans.
A new technique captures pairs of RNAs at the same time creating an association. The two types of RNAs in the technique are messenger RNAs creating the protein and the exact microRNA that is affecting it. One study uses mutations involved in Rett syndrome, a devastating brain disease related to autism. Some particular microRNAs were found in the cerebellum related to tags placed on DNA that specifically stimulated these microRNAs. These were related to the vital factor BDNF (brain derived neurotrophic factor), which stimulates new brain cells and improvement in treatment of depression, as well as memory. BDNF has also been associated with Rett syndrome and autism. These converged in a region related to the same miR-132.
Stress produces behavioral effects for generations of mice. The hypothalamic-pituitary-adrenal axis was altered as well as particular microRNAs and messenger RNAs in later generations. Sperm also showed microRNA changes. Another study showed that maternal separation increased anxiety and depression symptoms and altered insulin metabolism. These also showed changes in microRNA as the other study. Injecting this microRNA altered other mice to produce the same behavior and metabolism changes. This shows that microRNAs might be involved in the inheritance of altered behaviors.
Altering microRNA Production
Early research altered enzymes that produced microRNAs. But, in brain studies this killed neurons. Now, manipulating exact microRNAs has allowed better results. One genetic alteration of the production of a microRNA in gene 8 (DGCR8) has been associated with schizophrenia. Deleting this gene leads to increased risk. In a mouse model, another deletion had a similar effect. Both caused less mature microRNAs in the brain and showed unusual hyperactive behaviors and memory issues.
Another similar study in humans found decrease of miRNA in the pre frontal cortex with schizophrenia after death. A second study study found an increase of different microRNAs in different regions. This particular gene finding in humans related to only 2% of schizophrenic patients. Complex findings of this type were found for miR-185.
In knockout mice, two types of microRNAs were introduced to study miR-16 and its relation to serotonin transporters and effects of antidepressants. More miR-16 was found in noradrenergic cells than serotonin cells. Treatment reduced microRNAs and the serotonin transporter. Complex interactions with these two brain regions were found related to the microRNA.
miR-128b was sent by virus into the infra limbic PFC (pre frontal cortex near the limbic system) in experiments related to fear. With this study, fear in animals could be increased and decreased in different regions by altering microRNAs.
In adult mice, stress altered microRNAs in amygdala with increasing amounts of miR-34. When more miR-34 was introduced using a virus, anxiety decreased even with extreme stress. MicroRNA was noted to have interactions with receptors with the stress related corticotropin releasing hormone. This microRNA was shown to regulate responses to stress.
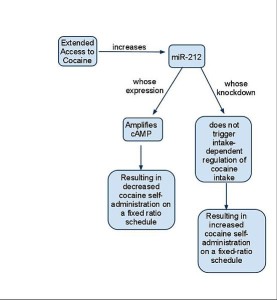
Another study showed that miR-212 is related to effects of cocaine. With excessive use of cocaine, there was more of microRNA in dorsal striatum. Antagonists reversed this effect. This microRNA increased CREB and other receptors (CREB is a vital factor that affects gene networks named from cAMP response element-binding protein). The effects of these receptors were found to be similar to the effects of the microRNA given.
Other studies found that miR-185 is specifically relevant to neurons and particularly their Golgi related genes. This affects creation of vesicles in the neuron. Also dendrite spines were disrupted when large amounts of the microRNA were given and the opposite effect occurred with too little.
Psychiatric Illness and microRNA
 It is still very difficult to study the biology of psychiatric illness since there are no definite biological markers or mechanisms known for the various illnesses. There are a lot of possible genetic associations that are not precise. A lot more is known about microRNAs in cancer. In brain diseases there is not just one site that is important, but effects can occur in many parts of the brain at once, making study much more complex.
It is still very difficult to study the biology of psychiatric illness since there are no definite biological markers or mechanisms known for the various illnesses. There are a lot of possible genetic associations that are not precise. A lot more is known about microRNAs in cancer. In brain diseases there is not just one site that is important, but effects can occur in many parts of the brain at once, making study much more complex.
Despite these difficulties, clearly microRNAs are relevant to psychiatric illness and over time more will results will be found.