 Vesicles containing neurotransmitters at synapses are well known, but mechanisms explaining their speed are still not clear. Very recently, neurons have been found to use many other types of vesicles to communicate with other cells, not at the synapse. Myelin patterns have been found to be much more variable than previously thought. These new types of vesicles are secreted in areas of the axon without myelin and at the cell body. Information sent to other cells in these vesicles includes genetic code, many kinds of RNAs, proteins and many other factors. Messages send important information to the other cells, such as astrocytes, microglia, oligodendrocytes, many immune cells and even muscle. Transmitted information can cause dramatic changes in behavior of the receiving cells. One type of information is where myelin should be built and another about how microglia and astrocytes should function. This post will update the recent research about the many different ways extra cellular vesicles in the brain are used.
Vesicles containing neurotransmitters at synapses are well known, but mechanisms explaining their speed are still not clear. Very recently, neurons have been found to use many other types of vesicles to communicate with other cells, not at the synapse. Myelin patterns have been found to be much more variable than previously thought. These new types of vesicles are secreted in areas of the axon without myelin and at the cell body. Information sent to other cells in these vesicles includes genetic code, many kinds of RNAs, proteins and many other factors. Messages send important information to the other cells, such as astrocytes, microglia, oligodendrocytes, many immune cells and even muscle. Transmitted information can cause dramatic changes in behavior of the receiving cells. One type of information is where myelin should be built and another about how microglia and astrocytes should function. This post will update the recent research about the many different ways extra cellular vesicles in the brain are used.
Cellular Communication
 Cells use many types of signals for communication. It has been surprising to learn how many ways cells communicate with other and the complexity of the back and forth chatter. As well as secretion of well-known neurotransmitters and cytokines, most cells also use nanotubes between cells and vesicles to send information in the form of genetic molecules, proteins and other factors.
Cells use many types of signals for communication. It has been surprising to learn how many ways cells communicate with other and the complexity of the back and forth chatter. As well as secretion of well-known neurotransmitters and cytokines, most cells also use nanotubes between cells and vesicles to send information in the form of genetic molecules, proteins and other factors.
A previous post described the very complex rapid release of vesicles with neurotransmitters at the synapse. Another post described how critical vesicles (exosomes) are to communities of cancer cells for their survival and spread. The use of vesicles to send information back and forth between neurons and other brain cells has been mentioned already. But, the full extent of this extra cellular vesicle communication with exosomes and micro vesicles is just now being described. This newly identified vesicle communication has been now seen for neurons with astrocytes and with oligodendrocytes about providing myelin for global neuroplasticity. Further research shows vital signals with microglia increase the spread of degenerative diseases and cancer.
Extra Cellular Vesicles
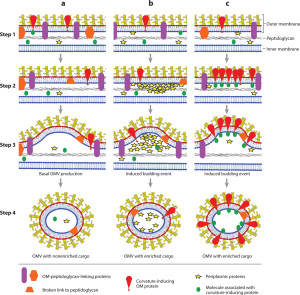 Extra cellular vesicles (EVs) are sent from the cell’s membrane into the extracellular region between cells. Micro vesicles are between 100 nm (nano meter) and 1 μm (micro meter) in diameter and the smaller exosomes are 30 to 100 nm. (milli, micro, nano are each 1000th of the other). Vesicles are formed by taking a piece of the cell membrane or are produced from large multi vesicular bodies (MVBs). The difference derives from the location they are produced in secretory cellular pathways between Golgi, endoplasmic reticulum and lysosomes. Lysosomes destroy or recycle used vesicle material. This pathway was described in a post on lipid pathways.
Extra cellular vesicles (EVs) are sent from the cell’s membrane into the extracellular region between cells. Micro vesicles are between 100 nm (nano meter) and 1 μm (micro meter) in diameter and the smaller exosomes are 30 to 100 nm. (milli, micro, nano are each 1000th of the other). Vesicles are formed by taking a piece of the cell membrane or are produced from large multi vesicular bodies (MVBs). The difference derives from the location they are produced in secretory cellular pathways between Golgi, endoplasmic reticulum and lysosomes. Lysosomes destroy or recycle used vesicle material. This pathway was described in a post on lipid pathways.
Some of the vesicles are used inside the cell, some are sent to the cell’s membrane to be sent outside the cell and some are recycled for material. Part of this secretory pathway is a major large protein complex, called the endosomal sorting complex for transport (ESCRT). The ESCRT is required for transport. Other secretory pathways are generally for lipids within the cell and for forming vacuoles and lysosomes.
Another type of vesicle is sent from the cell when it is committing programed suicide through apoptosis. These are not used for communication but rather an orderly breakdown of the cell as it is eaten by phagocytes.
Micro Vesicles
 Micro vesicles are formed in the plasma membrane by lipid rafts. Lipid rafts surround material and secrete it from the cell. Lipid rafts are particular complexes in the membrane that are, also, used for secretion of neurotransmitters at synapses. Some of the lipids used to form the vesicles are different in microglia. They are also unique in micro vesicles, exosomes and apoptotic vesicles.
Micro vesicles are formed in the plasma membrane by lipid rafts. Lipid rafts surround material and secrete it from the cell. Lipid rafts are particular complexes in the membrane that are, also, used for secretion of neurotransmitters at synapses. Some of the lipids used to form the vesicles are different in microglia. They are also unique in micro vesicles, exosomes and apoptotic vesicles.
Exosomes
 Exososomes are also called intraluminal vesicles (ILVs). They exist inside of cells and contain unique lipids such as ceremide, which allows it to be built at the lipid rafts. Specialized membrane regions, called microdomains, organize production of signaling molecules and vesicles. The lipid rafts float in the membrane, but are much more ordered than the general membrane and can produce these unique vesicles.
Exososomes are also called intraluminal vesicles (ILVs). They exist inside of cells and contain unique lipids such as ceremide, which allows it to be built at the lipid rafts. Specialized membrane regions, called microdomains, organize production of signaling molecules and vesicles. The lipid rafts float in the membrane, but are much more ordered than the general membrane and can produce these unique vesicles.
In compartments of the cell, a narrow space limits the size of the vesicles. Golgi provides proteins for the vesicle. Vesicles can fuse with the outside membrane and release the contents without a vesicle. Another type fuses with a lysosome and sends the contents to be broken down. Even though micro vesicles and exosomes are different, it is not yet clear that they have any differences in protein types. There are many different complex versions of ESCRT complexes that are used in a variety of circumstance, such as providing covers for many different viruses as they leave the cell’s membrane.
Cargoes In EVs
 Vesicles can carry RNA, lipids and even large proteins. From a research standpoint, it is hard to distinguish the various types of vesicles to define exactly what cargoes they have. Most transport inside of the cell, such as along the axon, or in the secretory pathways, involves tags on the molecules that describe their destination. This has been difficult to discover thus far in vesicles and it is very complex. Research on this appears in several available sources, Vesiclopedia and EVpedia. Another complication is that each type of cell has different versions of the EVs and other cells respond only to certain ones with special receptors. Also, there have been different definitions of exosomes and microvesicles.
Vesicles can carry RNA, lipids and even large proteins. From a research standpoint, it is hard to distinguish the various types of vesicles to define exactly what cargoes they have. Most transport inside of the cell, such as along the axon, or in the secretory pathways, involves tags on the molecules that describe their destination. This has been difficult to discover thus far in vesicles and it is very complex. Research on this appears in several available sources, Vesiclopedia and EVpedia. Another complication is that each type of cell has different versions of the EVs and other cells respond only to certain ones with special receptors. Also, there have been different definitions of exosomes and microvesicles.
EVs are, also, present in CSF and in blood, as part of normal communication.
ESCRT Complex in the Membrane

ESCRT or endosomal sorting complex for transport was first discovered related to cargoes sent to lysomes for breakdown of debris. A small region where the lumen is narrow forms an inward limited vesicle from the membrane forming a MVB and ILV. ESCRT then chooses what will go into the new vesicle. It, also, closes the vesicle. There are many different versions of ESCRTs and many different cellular functions are based on them.

ESCRT are a very complicated set of 100 different large proteins that are in five different sub units. Different species have different versions. There are many associated proteins as well. One type in metazoans uses ubiquitin for tagging of cargo. Many processes use multiple ESCRTs at the same time along with ubiquitin tagging. Some uses of ESCRTs are not stable, but change during the procedures, such as EXCRT-III. This one forms a very long thin structure that creates a coil around the vesicle where it is being cut from the internal membrane. This type is, also, used for the budding of some viruses, such as HIV.
 In the membrane, the lipid PI3P (lipid phosphatidyl-inositol 3 phosphate) is attracts the complex. This first molecular complex tags proteins with ubiquitin. ESCRT-0 binds ESCRT1, ESCRT2 and together they start separating off vesicles from the multi vesicle body membrane. It uses the ubiquitin tags to sort and identify the various vesicles that are made.
In the membrane, the lipid PI3P (lipid phosphatidyl-inositol 3 phosphate) is attracts the complex. This first molecular complex tags proteins with ubiquitin. ESCRT-0 binds ESCRT1, ESCRT2 and together they start separating off vesicles from the multi vesicle body membrane. It uses the ubiquitin tags to sort and identify the various vesicles that are made.
Exosomes have a different protein and lipid composition from other extracellular vesicles. This composition varies based on many factors. Exosomes often carry immune molecules, cytoskeleton molecules and cellular signals.
The mechanisms that build each type of vesicle is extremely complex and just now being researched. Data will be provided as it is discovered. For now, the rest of post discusses the particular vesicles and their functions in the brain.
EVs In The Brain
 Neurons secrete exosome sized EVs when stimulated with electrical action potentials, calcium channels, and molecules that block GABA or stimulate AMPA and NMDA. In one experiment fragments of tetanus toxin were sent to the neuronal surface. The toxin was brought into the cell and then released in vesicles from the neuron. Some showed transmission to other neurons. A brain tumor cell sent it to glia. One type of vesicle was found with receptors that were being discarded outside of the cell.
Neurons secrete exosome sized EVs when stimulated with electrical action potentials, calcium channels, and molecules that block GABA or stimulate AMPA and NMDA. In one experiment fragments of tetanus toxin were sent to the neuronal surface. The toxin was brought into the cell and then released in vesicles from the neuron. Some showed transmission to other neurons. A brain tumor cell sent it to glia. One type of vesicle was found with receptors that were being discarded outside of the cell.
Many kinds of microRNAs are sent in these vesicles. During neuroplasticity, microRNAs are discarded in vesicles from the cell when their effects of silencing specific genes are too strong. Specific microRNAs sent to astrocytes produce an increase in specific transporters that take up glutamate from outside of the cell. This has significant effects on the glutamate signaling.
Oligodendrocytes Use Vesicles
 A recent post on global neuroplasticity from myelin showed that communication between neurons and oligodendrocytes all over the brain is critical in determining the speeds of long range circuits so they can be coordinated to arrive at synapses at the correct moment for decision making. Vesicles are sent from axons that are not near the synapses to the oligodendrocytes. These carry proteolipid protein (PLP) and other myelin proteins critical for making myelin. They, also, include special proteins to help avoid oxidative damage. These are triggered by glutamate signals from neurons. The mechanism included calcium increase in the oligodendrocyte receptors for NMDA and AMPA.
A recent post on global neuroplasticity from myelin showed that communication between neurons and oligodendrocytes all over the brain is critical in determining the speeds of long range circuits so they can be coordinated to arrive at synapses at the correct moment for decision making. Vesicles are sent from axons that are not near the synapses to the oligodendrocytes. These carry proteolipid protein (PLP) and other myelin proteins critical for making myelin. They, also, include special proteins to help avoid oxidative damage. These are triggered by glutamate signals from neurons. The mechanism included calcium increase in the oligodendrocyte receptors for NMDA and AMPA.
Oligodendrocytes sent vesicles that are received by microglia and neurons. CRE recombinase enzyme was picked up from a vesicle and it helped neurons respond to cell stress. Special oligodendrocyte exosomes signal stem cells to stop making particular additional oligodendrocytes and myelin. This communication inhibits myelin from being made inappropriately or before they are near the neurons.
Some of the oligodendrocyte and neuron signals include PLP and are picked up by microglia using micro pinocytosis. This did not trigger microglia immune function, but rather was used as a way for the microglia to help rid the oligodendrocyte of cargo.
Vesicle Messages To Muscles
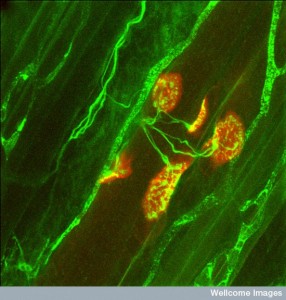 The extremely complex back and forth communication between neurons and muscles was described in a previous post. Exosomes have been found to be critical in this process. Far from active zones, vesicles are observed to fuse with the muscle away from the synapse site. These vesicles contain specific enzymes used by the muscle. The complex junction called the sub synaptic reticulum forms a special tube and this is where the exosomes travel to the muscle and trigger specific receptors.
The extremely complex back and forth communication between neurons and muscles was described in a previous post. Exosomes have been found to be critical in this process. Far from active zones, vesicles are observed to fuse with the muscle away from the synapse site. These vesicles contain specific enzymes used by the muscle. The complex junction called the sub synaptic reticulum forms a special tube and this is where the exosomes travel to the muscle and trigger specific receptors.
Schwann Cell EVs
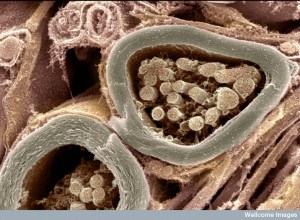 Schwann cells produce myelin in the peripheral nervous system. Remarkably, they are able to alter their differentiated state if there is damage and return to being stem cell to help with the regeneration of the tissue. These new stem cells release vesicles with particular receptors that respond to neurotrophin factors for regeneration. Neurons pick up these vesicles (exosomes) and they are used for the regeneration. Vesicle cargoes increased the rate of the new axon’s regeneration. They, also, helped with the remodeling and breakdown of the axon before regeneration by inhibiting the axon growth cone. Also, critical receptor molecules for BDNF were also taken into the axon.
Schwann cells produce myelin in the peripheral nervous system. Remarkably, they are able to alter their differentiated state if there is damage and return to being stem cell to help with the regeneration of the tissue. These new stem cells release vesicles with particular receptors that respond to neurotrophin factors for regeneration. Neurons pick up these vesicles (exosomes) and they are used for the regeneration. Vesicle cargoes increased the rate of the new axon’s regeneration. They, also, helped with the remodeling and breakdown of the axon before regeneration by inhibiting the axon growth cone. Also, critical receptor molecules for BDNF were also taken into the axon.
Microglia EVs
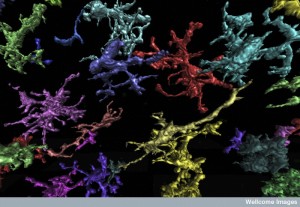 Microglia have been found to actively use EVs as well, not just to help eliminate debris. Stimulated by ATP from astrocytes on their surface receptors, microglia sent EVs with complex cargos. The vesicle included the precursor protein of cytokine interleukin-1β (IL-1β), as well as critical enzymes and receptors. The purpose of these vesicles is to release cytokines by action of the enzyme cutting the precursor protein. These vesicles are sent just in case they are needed, if tissue damage is found. As soon as damage is found, then the vesicles alter the precursor into the active cytokine, which is critical to stimulate the inflammatory response.
Microglia have been found to actively use EVs as well, not just to help eliminate debris. Stimulated by ATP from astrocytes on their surface receptors, microglia sent EVs with complex cargos. The vesicle included the precursor protein of cytokine interleukin-1β (IL-1β), as well as critical enzymes and receptors. The purpose of these vesicles is to release cytokines by action of the enzyme cutting the precursor protein. These vesicles are sent just in case they are needed, if tissue damage is found. As soon as damage is found, then the vesicles alter the precursor into the active cytokine, which is critical to stimulate the inflammatory response.
Microglia vesicles are, also, used to regulate the neuron’s action potential. Cargoes from the microglia stimulated neurons to fire with neurotransmitter release at the synapse. The neuron was also stimulated to make more ceremide and other special lipids for the creation of more vesicles. This also stimulated more production of the SNARE complex, a large multi protein structure that helps with vesicle launching from the membrane.
 Microglia are able to regulate the balance of neuronal stimulatory and inhibitory signals. Their micro vesicles include cargos of endocannabinoids, critical lipid molecules that are involved in the regulation of synapses. Endocannabinoids are often on the surface of the vesicle and can trigger the CB1 receptor in GABA neurons, which inhibits the inhibitory action of GABA. The microglia vesicles at the same time can stimulate excitatory neurons.
Microglia are able to regulate the balance of neuronal stimulatory and inhibitory signals. Their micro vesicles include cargos of endocannabinoids, critical lipid molecules that are involved in the regulation of synapses. Endocannabinoids are often on the surface of the vesicle and can trigger the CB1 receptor in GABA neurons, which inhibits the inhibitory action of GABA. The microglia vesicles at the same time can stimulate excitatory neurons.
Some microglia micro vesicles send special immune signals similar to B lymphocytes and dendritic cells. They include complex cargo with receptors for class II MHC major histocompatibility complex, chaperone molecules, and other enzymes and receptors. One cargo includes an enzyme involved in opioid metabolism. They contain molecules related to Alzheimer’s. During large energy use of neurons when making an action potential, microglia send vesicles filled with lactate to use as an alternative energy source.
Several neurotransmitters such as serotonin stimulate receptors on microglia. These send vesicles with special enzymes related to breaking down insulin and amyloid–β. High levels of serotonin can decrease the amount of the amyloid–β.
Astrocyte EVs
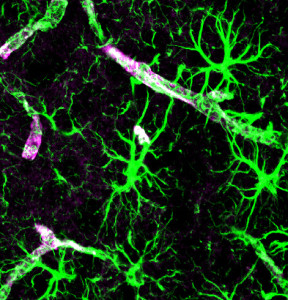 Astrocytes have many critical functions related to producing and maintaining synapses. They also help balance ions and the blood brain barrier. They secrete exosomes that include IL-1β when stimulated by ATP.
Astrocytes have many critical functions related to producing and maintaining synapses. They also help balance ions and the blood brain barrier. They secrete exosomes that include IL-1β when stimulated by ATP.
The communication between oligodendrocytes, astrocytes, microglia and neurons involves many different uses of EVs. Research shows that these messages affect the amount of activity that the neuron will produce, the responses to cellular and organism stress and repair and protection of the neuronal network.
EVs in Invertebrates
 More research has been able to be done on worms and flies than humans that shows prominent use of EVs for communication between cells in their nervous system.
More research has been able to be done on worms and flies than humans that shows prominent use of EVs for communication between cells in their nervous system.
In flies, the junction between the neuron and muscle has been widely studied. EVs control development of the neuro muscular junction during development both in the pre junction neuron and the post synaptic muscle. As muscles grow, communication is sent back and forth altering the junction through messages.
Communication occurs not at the junction but an adjacent area called the sub synaptic reticulum (SSR). Signals have proteins that are specially modified to become hydrophobic and therefore cannot just be secreted but rather need to be transported in a vesicle. With vesicles, the molecule can be taken right up to special receptors. Vesicles seem to use SNARE machinery that is similar to humans. Vesicles also are sent in response to the level of activity of the neuron, with many going out if there is greater frequency of action potentials.
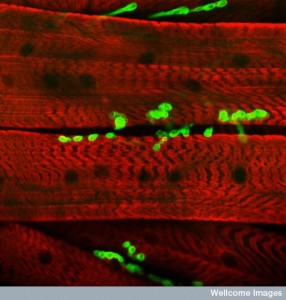 At the neuro muscular junction exosomes are exchanged as well. Vesicles from the neuron sends information that has direct effects on the muscles related to plasticity and development. One protein that is often used for regulation of both anterograde and retrograde transmission in the brain is released in these exosomes for the muscle, just like microglia. It is also used in the fetus and with cancer. Another particle sent in exosomes regulates how many glutamate receptors there are in the muscle junction.
At the neuro muscular junction exosomes are exchanged as well. Vesicles from the neuron sends information that has direct effects on the muscles related to plasticity and development. One protein that is often used for regulation of both anterograde and retrograde transmission in the brain is released in these exosomes for the muscle, just like microglia. It is also used in the fetus and with cancer. Another particle sent in exosomes regulates how many glutamate receptors there are in the muscle junction.
In worm sensory neurons cilia secrete vesicles with receptors. A complex transport mechanism for these vesicles lies along the flagella including the motor kinesin. Vesicles communicate with other worms related to mating behavior and increase their movement.
EVs In Disease
 A previous post described how mis folded proteins in the brain can be transmitted by vesicle transport between brain cells. They are, also, used in brain cancers (see post on Cancer and Exosomes) and in inflammation. But, they, also, send information for protection of neurons and are beginning to be used in treatment.
A previous post described how mis folded proteins in the brain can be transmitted by vesicle transport between brain cells. They are, also, used in brain cancers (see post on Cancer and Exosomes) and in inflammation. But, they, also, send information for protection of neurons and are beginning to be used in treatment.
Prions are, also, transmitted as well in vesicles. (See post on Transmission of of Prions). Prion behavior is found in many other mis folded proteins that cause disease including amyloid-β and tau in Alzheimer’s; α-synuclein in Parkinson’s disease; and TAR DNA-binding protein (TDP43), copper zinc superoxide dismutase 1 (SOD1) and other proteins in amyotrophic lateral sclerosis and fronto temporal degeneration. Now, it has been found that all of these are packaged and transmitted in vesicles. This is difficult research because, a very small amount of mis folded protein can start this process, particularly prions. Also, it is possible that different forms of the proteins are sent in vesicles such as folded, unfolded and clumped (see post on the role of tau in brain disease). In cancers, vesicles can come from dying cells to further the cancer community.
It has not been established that vesicles are definitely part of the cause of Alzheimer’s. But, amyloid plaques have markers for exosomes. Other research points to exosomes being used as a protection for the cell to remove amyloid-β. Microglia in their reactive form in inflammation form micro vesicles that send material making amyloid soluble and increases Alzheimer’s pathology. In this scenario, micro vesicles increase Alzheimer’s and the exosomes do the opposite. Another study showed that abnormal tau is transmitted by exsosomes from microglia.
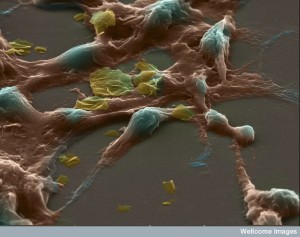 As a previous post on cancer noted, cancers use an increased amount of vesicles for communication between the communities of cells. What is interesting is that among cancer cells, the contents of vesicles contain material that is altered from normal. These proteins can help a cancer grow in different ways and are called transforming proteins. They, also, contain RNAs that can alter cells and various unique histones.
As a previous post on cancer noted, cancers use an increased amount of vesicles for communication between the communities of cells. What is interesting is that among cancer cells, the contents of vesicles contain material that is altered from normal. These proteins can help a cancer grow in different ways and are called transforming proteins. They, also, contain RNAs that can alter cells and various unique histones.
Glioblastomas use stem cells to build the tumor and use vesicles to increase survival, movement and reproduction of other cells. Studies show that contents of vesicles alter receptors and pathways. When cancer cells are injected into another animal, they produce vesicles that start the new cancers in the new cell. In order for breast cancer cells to cross the blood brain barrier to form metastasis in the brain, they send specific microRNAs in vesicles that degrade the choroid cells at the blood brain barrier.

Microglia and astrocytes use vesicles as part of neuro inflammation. Neuro inflammation was described in another post as a complex form of neuroplasticity, where neurons produce all of the symptoms and signs of inflammation in various measured ways. In multiple sclerosis, cytokines are sent in the vesicles (cytokine IL-1β, interferon-γ, tumour necrosis factor (TNF) among others) to further inflammation. These vesicles also include proteins that can damage the blood brain barrier altering the tight junctions and extra cellular matrix. This allows T cells and other immune cells to cross into the brain without permission of the choroid cells (see post on Choroid Cells). When neurons are injured, astrocytes are activated and produce a large number of vesicles. Oligodendrites get into the act by stimulating microglia to further the inflammation. Patients with multiple sclerosis have a larger than normal amount of vesicles in the blood and CSF.
There are many other examples of vesicles used in inflammation. Pattern receptors normally pick up microbes and trigger inflammation responses. In the brain pattern recognition receptors on all three glia cells pick up injured cells and produce more cytokines that can increase damage. Many different inflammatory vesicles are produced in the degenerative diseases of Alzheimer’s, Parkinson’s and ALS.
Protecting the Brain
 As well as spreading disease, vesicles also protect the brain. They send materials to rebuild after injury, disease and stress. They take toxic material to be removed including amyloid. They fight against prions. They help avoid damage from oxidation. Oligodendrocytes produce many molecules to help with stress from oxidative molecules including SOD1 and these are sent in vesicles to neurons. They send signals to avoid apoptosis and necrosis and help increase neuronal metabolism. HSPs (heat shock proteins) help as signals against stress and as chaperones. They were thought to only work inside of cells, but now it is shown that vesicles transmit them to help other cells. Many different brain cells send HSP in response to stress.
As well as spreading disease, vesicles also protect the brain. They send materials to rebuild after injury, disease and stress. They take toxic material to be removed including amyloid. They fight against prions. They help avoid damage from oxidation. Oligodendrocytes produce many molecules to help with stress from oxidative molecules including SOD1 and these are sent in vesicles to neurons. They send signals to avoid apoptosis and necrosis and help increase neuronal metabolism. HSPs (heat shock proteins) help as signals against stress and as chaperones. They were thought to only work inside of cells, but now it is shown that vesicles transmit them to help other cells. Many different brain cells send HSP in response to stress.
Vesicles work against aging also. MicroRNAs are sent by oligodendrocytes to produce more stem cells for increased myelination during injury.
Treatment with EVs
 Exosomes are found in all parts of the body and in all fluids and are increasingly part of disease diagnoses. They can be possible vehicles for treatment. Some exosomes pass through the blood brain barrier and can carry RNAs of various kinds and mediations. They do not trigger many immune reactions. They can be sent directly to specific types of cells with identification tags.
Exosomes are found in all parts of the body and in all fluids and are increasingly part of disease diagnoses. They can be possible vehicles for treatment. Some exosomes pass through the blood brain barrier and can carry RNAs of various kinds and mediations. They do not trigger many immune reactions. They can be sent directly to specific types of cells with identification tags.
Many stem cells send particularly useful messages in exosomes that could be used for therapies. Vesicles can be produced by stem cells in cultures when stimulated by various factors. They produce very specific microRNAs that increase healing and neuroplasticity in an ischemic damaged area. Another type of exosome from a stem cell sends an enzyme that can degrade amyloid-β and can rid it from inside cells when they take up the exosome. It can also workand in the extra cellular matrix.
Other examples:
- Exosomes from immune dendritic cells that are stimulated by interferon-gamma produce microRNAs such as miR-219 that stimulates stem cells to produce more oligodendrocytes and more myelin production.
- Exosomes produced by macrophages produce enzymes that are anti oxidants and have been useful with Parkinson’s patients.
- Another type is filled with siRNAs that stop beta-secretase enzyme to help Alzheimer’s.
Extra Cellular Vesicles in the Brain
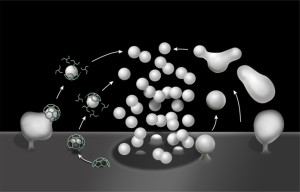 As more and more communication is found between all types of cells, important new messages have been found that are used by neurons. Vesicles used at synapses are well known. But, recently many other types of vesicle messages have been found. Similar messages have been found using nanotubes. Both these new vesicles and nanotubes are very small and have only recently been seen with electron microscopes. But, the research is very arduous and the information is only now coming out.
As more and more communication is found between all types of cells, important new messages have been found that are used by neurons. Vesicles used at synapses are well known. But, recently many other types of vesicle messages have been found. Similar messages have been found using nanotubes. Both these new vesicles and nanotubes are very small and have only recently been seen with electron microscopes. But, the research is very arduous and the information is only now coming out.
Advanced communication from neurons occurs with astrocytes, microglia, oligodendrocytes, many immune cells and even muscle cells. Where is the global understanding of the situation that allows a cell to communicate with another cell at great distance? Can anyone think this is random? Can anyone think these cells are not intelligent?
Where is the direction for this? Isn’t it reasonable to consider some form of mind interacting with these cells?