 Some think viruses are not alive. It is, therefore, very surprising that they can evade elaborate cellular mechanisms used to find and destroy them. Search and destroy mechanisms of the cell and counter attacks from viruses are very complex. Cells use many sensors to find DNA and RNA that is not where it is supposed to be. When found, other mechanisms are triggered to get rid of it. Major cellular tools are pattern recognition receptors with enormous numbers of variations allowing detection of any foreign molecule. Viruses use many techniques to avoid being caught. They are able to hide from these receptors by isolating its DNA or RNA or modifying it. Viruses can interfere with processes that pattern receptors use by segregating them, changing their adaptor molecules, and altering metabolism to destroy them.
Some think viruses are not alive. It is, therefore, very surprising that they can evade elaborate cellular mechanisms used to find and destroy them. Search and destroy mechanisms of the cell and counter attacks from viruses are very complex. Cells use many sensors to find DNA and RNA that is not where it is supposed to be. When found, other mechanisms are triggered to get rid of it. Major cellular tools are pattern recognition receptors with enormous numbers of variations allowing detection of any foreign molecule. Viruses use many techniques to avoid being caught. They are able to hide from these receptors by isolating its DNA or RNA or modifying it. Viruses can interfere with processes that pattern receptors use by segregating them, changing their adaptor molecules, and altering metabolism to destroy them.
So Much With So Few Genes and Proteins
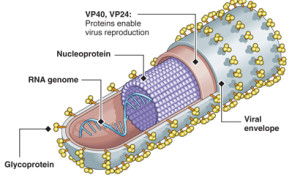 Previous posts have described elaborate behavior of viruses that have only a hand full of genes and proteins. With this small number, they are able to out maneuver human cells that are many thousands of times larger and more complex. They can somehow block receptors, signals and pathways that would otherwise attack them.
Previous posts have described elaborate behavior of viruses that have only a hand full of genes and proteins. With this small number, they are able to out maneuver human cells that are many thousands of times larger and more complex. They can somehow block receptors, signals and pathways that would otherwise attack them.
Ebola has 7 genes and proteins but can build a huge complex vessel for travel. Attachment to cells involves many techniques that involve evasion techniques. It evades sensors and even creates a decoy to confuse the immune system. It can take over protein rafts in the cell membrane and use it for their own purposes.
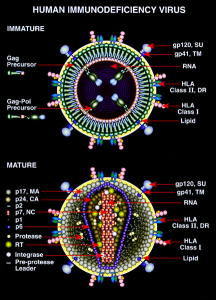 HIV has 9 genes and proteins. It travels with several proteins that are used to evade defenses and enter immune cells designed to destroy them.
HIV has 9 genes and proteins. It travels with several proteins that are used to evade defenses and enter immune cells designed to destroy them.
Herpes has 70 genes and a very complex lifestyle. It takes over transport machinery in neurons and travels up and down axons to and from the skin. It changes its behavior several times becoming active and quiescent. It has many ways to evade cells.
Perhaps most remarkable is how much they can do with so few genes and proteins. Each protein has many different complex functions. It is not at all clear how they can do so much with so little.
Pattern Receptors Protect Cell From Virus
The intricate workings of pattern receptors were discussed in a previous post. This post focuses on those that are used for virus RNA and DNA and the virus response. These include RIG-I-Like receptors or RLRs, interferon-γ (IFNγ)-inducible protein 16 (IFI16), and cyclic GMP-AMP synthase (cGAS).
With intensive large-scale research into HIV and Hepatitis C, medications were discovered that inhibited vital functions of these viruses. But, there are a very large number of other significant and dangerous viruses where little is known and there is not enough money to do as much research as HIV. Viruses become resistant to drugs and new ones are appearing that are threatening, such as Ebola and Zika.
Viruses must use cell machinery. Cells have developed elaborate defensive mechanisms. So, new viruses are more sophisticated in evading these defenses. Special genes make the first line of defense of pattern recognition receptor (PRR) proteins. Two major types of PRRs are those that recognize pieces of virus outside of cells and those that recognize them inside of the cells.
 For recognition outside of the cell, PRRs are on the cell’s membranes including Toll like receptors or TLRs and C type lectin receptors or CLRs. CLRs bind to microbe sugar molecules on many immune cells such as dendritic cells and macrophages.
For recognition outside of the cell, PRRs are on the cell’s membranes including Toll like receptors or TLRs and C type lectin receptors or CLRs. CLRs bind to microbe sugar molecules on many immune cells such as dendritic cells and macrophages.
Inside of cells, PRRs are in cytoplasm and the nucleus. These include NOD like receptors (NLRs). These are called nucleotide-binding domain (NBD) and leucine-rich repeat (LRR). Another class is RLRs. A third category is DNA sensors such as cGAS and IFI16. These exist in most cells. These PRRs start signaling cascades that include many kinases and other enzymes. The pathways end with transcription factors that alter genetic networks. These signaling pathways lead to powerful cytokines such as interferon I and II that stimulate immune responses. They also stimulate chemokines,that are cytokines that can attract more cells to fight at a particular location.
These cytokines trigger a large number of genes (hundreds or thousands) called IFN (interferon) stimulated genes or ISGs. These produce many different proteins that attack the lifestyle of viruses at many different places.
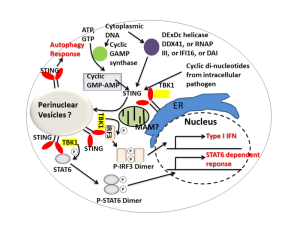
There are two large major adaptor molecules that provide systems of communication between the immune pathways and pattern recognition signals from cells. Viruses can grab onto the adaptors that trigger immune responses and modify them as well as the receptors.
A major adaptor between PRRs and immune response is STING, named for “stimulator of IFN (interferon) genes”. The other is connected with mitochondria and is called MAVS or “mitochondrial antiviral signaling protein”. MAVs can be found connected to mitochondria, but also to large vesicles called peroxisomes. Peroxisomes are part of the secretory system of vesicles that connect with lysosomes to clean debris and microbes from the cell. A previous post described how bacteria could take over these vesicles, particularly vacuoles. Viruses, much smaller than these bacteria, manipulate them. They are able to relocate PRR receptors to the vesicle so they are hidden and won’t work.
Virus Evasion

Very recently, an ingenious evasion technique of HIV was discovered. HIV uses this unusual manipulation to enter the cell’s nucleus. Normally, a motor protein KIF5B transports cargoes away from the nucleus. HIV is somehow able to hijack this motor (how can they with only a small number of genes). HIV makes the protein tear off pieces of the nuclear envelope (pieces are proteins called Nup358. making the nuclear pore much larger. When it is large enough, HIV enters the nucleus through the pore. It is quite amazing that HIV can do this.
Many other techniques have been recently discovered. Clever viruses with only a handful of genes are able to avoid these sensors with many mechanisms as well as inhibiting the very production of the PRRs and their pathways.
- One technique is to hide their RNA and DNA from the cell mechanisms.
- A second is to interfere with the production of the PPRs by affecting how they are modified with tags. Also, they also alter adaptor molecules with tags.
- The third is by cutting the PPRs or altering them by stimulating their metabolism. This includes these processes related to their adaptor molecules as well.
- The fourth is to take the PPRs and segregate them in compartments where they are not useful.
How Do Cells Recognize DNA and RNA from Viruses
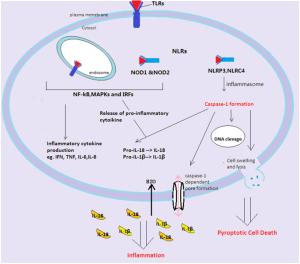
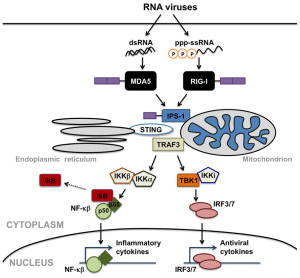 Cells recognize RNA and other products of transcription and stimulate interferon. NLR recognition receptors recognize single strands of RNA and produce an inflammasome, a large multi protein complex that produces and regulates inflammation.
Cells recognize RNA and other products of transcription and stimulate interferon. NLR recognition receptors recognize single strands of RNA and produce an inflammasome, a large multi protein complex that produces and regulates inflammation.
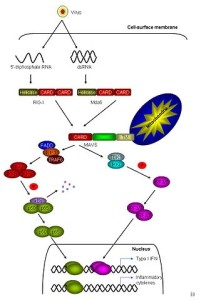
There are three major RLR pattern receptors that have very complex names since they are named from their first discovery in different situations. These include RIG-I, MDA5, and LGP2. RNA binds to particular places on each receptor such as the helicase region and the carboxyl-terminal. Other regions are involved as well called tandem caspase domains (CARD – caspase activation and recruitment domain) which stimulates immune pathways.
It appears that for many viruses both RIG-I and MDA5 are necessary. RIG is needed for rhabdovirus, influenza, and others with negative RNA strand. It also recognizes positive strands of HCF and JEV virus. MDA5 recognizes picornavirus. Both are needed for dengue, West Nile and reovirus. In fact, some viruses produce material for one or the other, or both. RIG can also find DNA virus such as Epstein Barr, Kaposi herpes, herpes simplex and adenovirus.
 Tags on the PPRs such as RIG and MDA5 are important to trigger immune pathways. Phosphor tags occur when they are suppressed with no virus present. When RNA appears, the PPRs change their shape produced by multiple enzymes including ATPase and kinases. Then phosphorus tags are removed from the PPR.
Tags on the PPRs such as RIG and MDA5 are important to trigger immune pathways. Phosphor tags occur when they are suppressed with no virus present. When RNA appears, the PPRs change their shape produced by multiple enzymes including ATPase and kinases. Then phosphorus tags are removed from the PPR.
But further tagging is necessary. RIG is tagged by E3 ubiquitin in two paces. Then RIG combines with three other RIG molecules at the mitochondria membrane. Another ubiquitin enzyme can take off the tag to suppress the action. When activated, the protein that connects to the mitochondria starts to combine together into a large structure, which is in the form of a filament like a prion. This structure includes and attracts significant cytokines such as TNF (tumor necrosis factor) and others. These then trigger many different cytokines to fight the virus (NF-kB, IL-6, and 8) and many ISGs.
A further mechanism has recently been found where RIG and MDA5 can block virus reproduction.
DNA is sensed by a set of receptors working through an adaptor protein (STING). The most is known about cGAS and IFI16 sensors.
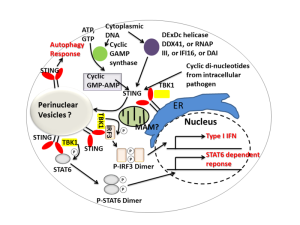 cGAS grabs onto DNA with double strands (dsDNA) from DNA viruses and retroviruses such as HIV. Through pathways they bind to STING. This stimulation can spread to other nearby cells through electrical synapses (gap junctions). STING attaches to another STING molecule and then is tagged by ubiquitin, which activates it, which moves it from the ER to the Golgi near the nucleus. There another phosphorus tag is put on, which produces NF-κB.
cGAS grabs onto DNA with double strands (dsDNA) from DNA viruses and retroviruses such as HIV. Through pathways they bind to STING. This stimulation can spread to other nearby cells through electrical synapses (gap junctions). STING attaches to another STING molecule and then is tagged by ubiquitin, which activates it, which moves it from the ER to the Golgi near the nucleus. There another phosphorus tag is put on, which produces NF-κB.
With IFI16, when triggered by virus DNA it grows into filaments and this binds to STING. This triggers cytokines and an inflammasome, which triggers more cytokines such as IL-1β. IFI16 can be in the nucleus as well. This is tricky because it means that it understands which DNA is from the “self” and which is not. It is not clear how it can know this. DNA viruses copy themselves in the nucleus, such as herpes, pailoma and polyma viruses. Somehow it triggers the STING mechanisms in the cytoplasm from the nucleus.
Viruses Evading RLRs
 Viruses have developed ways to inhibit the RLRs and the attachments to mitochondria. They also can inhibit parts of the pathways immune systems use. They can interfere with vital molecules that are used by many of these pathways such as TBK1, IRF3, IRF7 and NF-kb.
Viruses have developed ways to inhibit the RLRs and the attachments to mitochondria. They also can inhibit parts of the pathways immune systems use. They can interfere with vital molecules that are used by many of these pathways such as TBK1, IRF3, IRF7 and NF-kb.
RNA viruses copy in the cytoplasm. RLRs pick them up. Some viruses hide their RNA from the RLRs. Somehow, viruses are able to trigger a special compartment of the cell surrounded by membranes. This is similar to bacteria who take over large membrane surrounded organelles such as vacuoles. Another technique is to hide in the endoplasmic reticulum membranes, Golgi or mitochondria.
Dengue hides in the large folds of the endoplasmic reticulum membrane. There its doubled stranded RNA is hidden from RLRs and there is much less immune response.
Influenza virus reproduces in the nucleus to avoid RNA sensors. This is odd because the influenza A virus has eight DNA regions that would stimulate RIG. After entering the cell, flu virus travels quickly to the nucleus. One of the enzymes that flu carries with it is PB2. This virus is tightly packed by their own proteins because of special sections that create greater affinity.
 Ebola and Marburg have special proteins that block RIG from sensing it. Some viruses use cell proteins to hide from the sensors. Respiratory syncytial virus uses the cell’s protein, which binds to virus RNA to hide from RIG. Another protein hides parainfluenza virus from MDA5.
Ebola and Marburg have special proteins that block RIG from sensing it. Some viruses use cell proteins to hide from the sensors. Respiratory syncytial virus uses the cell’s protein, which binds to virus RNA to hide from RIG. Another protein hides parainfluenza virus from MDA5.
Some viruses actually change their own DNA and RNA to hide. Hantaan virus and Crimean Cogno hemorrhagic fever and Borna change a triple phosphate attachment on their gene to a single. This stops RIG from seeing it.
In another situation, Arenavirus has an unusual unpaired nucleotide that binds to RIG, but doesn’t activate it.
Lassa virus has a nucleoprotein that has a three dimensional fold that has enzyme activity that cuts free double stranded RNA thereby stopping the sensors.
Manipulating Modifications of Receptors
 RLR is dependent on tags (called post translational modification) of the ubiquitin variety and on molecules along the pathway. Proteins from the virus inhibit the enzyme that puts on the ubiquitin tags. Another version of this occurs with flu virus. Other viruses, RNA and DNA, directly remove the ubiquitin tag through enzymes. This includes SARS, foot and mouth disease, and others.
RLR is dependent on tags (called post translational modification) of the ubiquitin variety and on molecules along the pathway. Proteins from the virus inhibit the enzyme that puts on the ubiquitin tags. Another version of this occurs with flu virus. Other viruses, RNA and DNA, directly remove the ubiquitin tag through enzymes. This includes SARS, foot and mouth disease, and others.
Another mechanism uses the cell’s microRNAs. Viruses alter the amount of micro RNAs with protein interactions. A Picorno virus that causes brain disorders decreases the microRNA, miR-526a. This increases an enzyme that inhibits RIG.
One version of dengue recently in Puerto Rico interferes with ubiquitin tags of RIG.
It was mentioned above that phosphate tags keep RIG and MDA5 inhibited. Taking off these tags activates the PPRs and the immune response. Measles uses several proteins to block the dephosphorylation keeping down immune responses.
Nipah virus, which is similar to measles and causes brain disease in South Asia uses the same strategy. These use proteins that serve as decoys. They also use other proteins that interfere with the intermediaries in this tagging and untagging process.
Viruses Cut PRRs
The best way to stop PPRs is to destroy them. Many viruses make enzymes that cut the protein receptor. Polio makes one that cuts RIG and another for MDA5. Other viruses attack intermediary adaptors and cut them. This includes hepatitis A, rhinovirus and coxsackie virus. Flu virus degrades the intermediary in a different way. These produce a molecule that alters the mitochondrial membrane electrical potential which breaks apart the intermediary MAVS.
Another mechanism is to increase metabolism of the receptors. Hepatitis B makes a protein that binds to MAVS and starts its breakdown. This process involves affecting the ubiquitin tags. Polio cuts MDA5 indirectly. Also measles stimulates autophagy mechanisms to break down the receptors. This leads to death of mitochondria. SARS has a protein that goes to the mitochondria and alerts the ubiquitin mechanism.
A totally different mechanism comes from dengue where its proteins increases vesicles with enzymes that stops the interferon production.
Removing PRRs
 Several viruses produce proteins that bind directly to MDA5 and block its behavior. VP35 protein from Ebola (see post on Ebola), NS1 from flu and 4a proteins from MERS are able to bind to and therefore inhibit RIG and MDA5 in this way stopping interferon. Other proteins bind to RIG and remove it to a compartment.
Several viruses produce proteins that bind directly to MDA5 and block its behavior. VP35 protein from Ebola (see post on Ebola), NS1 from flu and 4a proteins from MERS are able to bind to and therefore inhibit RIG and MDA5 in this way stopping interferon. Other proteins bind to RIG and remove it to a compartment.
In the normal process, once the receptors find the viral molecules they must move to special organelles that can continue with the process. This involves moving with the viral piece to mitochondria and peroxisomes. Several molecules form viruses stop this movement and instead send them to different compartments where they won’t be effective.
Very surprisingly, viruses can create new compartments to place these receptors in. These are sometimes called viral inclusion bodies. Proteins from viruses binds to the receptor and moves it to the inclusion bodies. Another structure is like an endosome (a large sac with a membrane) that is stimulated by the infection with the virus.
Ubiquitin
A structure build by the cell to fight viruses is called the stress granule. These have RLR receptors and signaling. Polio and encephalomyocarditis virus cut proteins to stop the creation of these granules.
Stress granules are large accumulations of proteins and RNAs that appear when the cell is stressed. These RNAs have stopped working because of the stress and they are waiting to be restarted. Some of these are messenger RNAs that didn’t finish. These granules are near the endoplasmic reticulum. Another variety occur in the nucleus. While waiting at the stress granules RNAs are protected from destructions.
Another compartment viruses manipulate are peroxisomes. Peroxisomes are also called microbodies. They are organelles with a membrane that metabolize fatty acids, amino acids, and polyamines. They reduce hydrogen peroxide (hence the name peroxisome). They are important in energy production.
Another hiding place are endosomes, sometimes called multivesicular bodies. As they major they become more acid and then combine with lysosomes for destruction of material. Smaller vesicles bud off of big ones called lumenal vesicles and these become multi vesicular. As they fuse with lysosomes another compartment is created based on both.
Evasion of CGAS, IFI16 and STING
 The two most prominent DNA sensors in all cells are cGAS and IFI16 and both use STING. Retroviruses are able to inhibit cGAS and STING.
The two most prominent DNA sensors in all cells are cGAS and IFI16 and both use STING. Retroviruses are able to inhibit cGAS and STING.
HIV uses a single RNA strand transcribed in reverse into DNA inside the cell to place it in the cell’s genome. Few make it into the genes, the rest are open to the sensors. HIV somehow uses the cell’s enzymes to get rid of excess DNA to avoid a strong reaction. When these enzymes can’t be used then DNA accumulates, are sensed and immune response occurs. Proteins that surround HIV help evade cGAS.
HBV virus causes hepatitis, cirrhosis and liver cancer. The enzymes brought along by the virus block STING blocking its ubiquitin tags. SARS is an RNA virus similarly blocks the ubiquitin tags of STING. There are many other examples of this process with HPV papillomavirus, adenovirus through other protein actions. These are the same proteins that help stimulate cancers. Blocking immune responses and cancer responses appear to be highly related.
 Many DNA viruses have different ways to block STING. They block ubiquitin tags. Another mechanism actually makes a factor that regulates the powerful cytokine interferon. They appear to use many different techniques at the same time to avoid the sensors and the immune pathways that follow.
Many DNA viruses have different ways to block STING. They block ubiquitin tags. Another mechanism actually makes a factor that regulates the powerful cytokine interferon. They appear to use many different techniques at the same time to avoid the sensors and the immune pathways that follow.
HSV virus copies itself in the nucleus. One technique it uses is to attack and break down the sensor IFI16 in the nucleus. It does this by making its own attack enzymes and stimulating others as well. Other viruses create enzymes to attack and degrade STING. Several viruses make protease enzymes (enzymes that break down proteins) that attack both MAVs and STING.
Entirely different mechanisms make the sensors move to other compartments, in this case the multivesicular bodies involved in making secretory vesicles. In some cases the virus then uses some of the proteins of the sensors themselves.
Viruses evade DNA RNA Sensors
 It is quite surprising that tiny viruses with a handful of genes and proteins can manipulate these large immune structures such as pattern receptors pathways. Viruses maneuver in many different ways. They can take receptors to another compartment. They can attack the pathways and inhibit action. They can cut the receptors. They can perform these maneuvers on the adaptor molecules also, like STING and MAVS.
It is quite surprising that tiny viruses with a handful of genes and proteins can manipulate these large immune structures such as pattern receptors pathways. Viruses maneuver in many different ways. They can take receptors to another compartment. They can attack the pathways and inhibit action. They can cut the receptors. They can perform these maneuvers on the adaptor molecules also, like STING and MAVS.
How can a small stand of RNA and DNA have such a complex set of behaviors?