 Exciting new research about chronic pain was featured in the previous post. It describes the many cells that are not neurons producing varied types of chronic pain with unorthodox signaling pathways. These diverse cells that stimulate unique new signaling pathways that stimulate particular kinds of chronic pain includes astrocytes, microglia, cancer cells, tissue cells, lining cells, blood and immune cells, and even microbes. It is quite remarkable that so many different cells can produce pain syndromes in coordination with neuronal circuits.
Exciting new research about chronic pain was featured in the previous post. It describes the many cells that are not neurons producing varied types of chronic pain with unorthodox signaling pathways. These diverse cells that stimulate unique new signaling pathways that stimulate particular kinds of chronic pain includes astrocytes, microglia, cancer cells, tissue cells, lining cells, blood and immune cells, and even microbes. It is quite remarkable that so many different cells can produce pain syndromes in coordination with neuronal circuits.
Along with these new signaling pathways, new neuronal circuits related to chronic pain are being found as well. The previous post detailed the many cells that produce these unusual pain syndromes. This post describes newly discovered neuronal pain circuits in the spinal cord and elsewhere.
What Is Pain and Where Are Its Brain Circuits
 Pain is essential to let us know about trouble. Without pain circuits and being unaware of trouble, mortality is much greater. Pain has been defined as unpleasant sensations related to actual or potential tissue damage. Recent understanding of pain is much more complex with many neurons detecting pain from both the outside and internal tissues, as well as mental experiences. There are now many pathways that produce pain not caused by any specific damage, but through sensitization of distant circuits from pain caused by regional damage.
Pain is essential to let us know about trouble. Without pain circuits and being unaware of trouble, mortality is much greater. Pain has been defined as unpleasant sensations related to actual or potential tissue damage. Recent understanding of pain is much more complex with many neurons detecting pain from both the outside and internal tissues, as well as mental experiences. There are now many pathways that produce pain not caused by any specific damage, but through sensitization of distant circuits from pain caused by regional damage.
Information about pain is distributed among many different brain regions that are connected through the spinal cord. These circuits connect with cognitive, emotional, sensory, and movement brain centers.
Another new complication is that pain can be experienced as many different sensory experiences—burning, stabbing, throbbing, aching, hard touching, and more. These experiences can generalize to become broader experiences of suffering. Chronic pain can occur when the original problem continues, but also after it heals. This can occur through other circuits where the original pain experience itself produces other longer lasting types of pain. These chronic pain syndromes can be very debilitating with fewer treatment options. These recent discoveries of diverse cells and unusual circuits producing pain, often with new signaling molecules and neurotransmitters, hopefully will lead to completely new types of treatments.
Pain Signals From Periphery
 There are a surprising number of different pain receptor neurons and most are yet to be discovered. It is also not known how specific pain neurons produce particular types of pain. Some facts are known. Because they are being discovered in many different types of experiments, many of the names of these fibers are long and bizarre.
There are a surprising number of different pain receptor neurons and most are yet to be discovered. It is also not known how specific pain neurons produce particular types of pain. Some facts are known. Because they are being discovered in many different types of experiments, many of the names of these fibers are long and bizarre.
Several different sized neurons (with varied thickness, amount of myelin, and conduction abilities) pick up pain signals in the skin, tissues, and organs. They can signal from many different types of disturbance—toxins, chemicals, heat, cold, or mechanical damage. Most pain signals involve multiple receptors being triggered, such as several kinds of heat sensors (such as transient receptor potential channel vanilloid TRPV1 or TRPM3 and calcium activated chloride channel ANO1). If only one of these channels are eliminated, pain signals are usually slightly decreased. Cold also has multiple varied receptor channels (including TRPM8). Many types of pain receptors are not yet understood.
 Master skin regulator cells (keratinocytes – see post on Intelligent Skin Cells) are very important in modulating neurons for pain from the skin. One type of skin cell (Merkel cell) picks up mechanical disturbances (using receptor Piezo2s to slowly adapting neurons type 1 – SA1). The exact neurotransmitter molecules are not yet understood for these pain signals from the skin.
Master skin regulator cells (keratinocytes – see post on Intelligent Skin Cells) are very important in modulating neurons for pain from the skin. One type of skin cell (Merkel cell) picks up mechanical disturbances (using receptor Piezo2s to slowly adapting neurons type 1 – SA1). The exact neurotransmitter molecules are not yet understood for these pain signals from the skin.
Some neurons have been found to send signals for several different kinds of pain signals (polymodal neurons). Genetic studies show a nerve fiber for touch with mainly the channel Piezo2, but no have receptors for heat. Another neuron deals significantly with acute mechanical pain and ongoing mechanical pain but has no effect on heat (MRGPRD Mas-related G protein coupled receptor subtype D). Another, CGRP (calcitonin gene related peptide which is significant in trigeminal nerves and migraines), has some of the same properties as TRV and TRPM3 for heat, but not for mechanical pain. They also were highly related to cold and even itch.
Spinal Cord
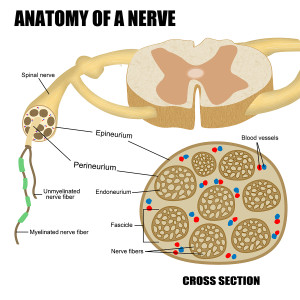 The Spinal cord center called the dorsal horn is where all pain fibers from the skin, tissues, and organs are organized and integrated. It was thought that these signals were then sent up the spinal cord to higher brain centers. Also, it has been known that many fibers come down from the higher centers that modulate this pain. It has only recently found out that the dorsal horn is much more complex and has many small micro circuits that are vital for different kinds of pain. These neurons that only travel from one part of the horn to another are called interneurons rather than projection neurons that travel a great distance to other centers. These interneurons can be stimulating and can be inhibiting.
The Spinal cord center called the dorsal horn is where all pain fibers from the skin, tissues, and organs are organized and integrated. It was thought that these signals were then sent up the spinal cord to higher brain centers. Also, it has been known that many fibers come down from the higher centers that modulate this pain. It has only recently found out that the dorsal horn is much more complex and has many small micro circuits that are vital for different kinds of pain. These neurons that only travel from one part of the horn to another are called interneurons rather than projection neurons that travel a great distance to other centers. These interneurons can be stimulating and can be inhibiting.
There is a lot to be learned about the primary sensory neurons that go to the centers in the spinal cord. The dorsal horn is divided into six layers called lamina. This is the place where sensory neurons send information to be synthesized. It is also the place where neurons from the higher brain, such as the cortex send information to modify the pain signals (supraspinal modulation).
 Much like the cortex layers, the dorsal horn is organized with anatomical columns from front to back. Two major well known pain fibers (C and Ad-nociceptive) go to the layers 1 and 2, which are most connected to signals of toxins and pain from the periphery. Ad fibers that are low threshold and Ab fibers go to layers 3 and 5, which are deeper into the dorsal horn and are mostly related to touch.
Much like the cortex layers, the dorsal horn is organized with anatomical columns from front to back. Two major well known pain fibers (C and Ad-nociceptive) go to the layers 1 and 2, which are most connected to signals of toxins and pain from the periphery. Ad fibers that are low threshold and Ab fibers go to layers 3 and 5, which are deeper into the dorsal horn and are mostly related to touch.
There are a significant number of small interneurons and there are a large number of neurons that travel from 1,3, 4, and 5 and go up and out of the spinal cord into the higher brain that greatly affects the many experiences of pain and its relation to emotion.
Making Pain Experiences – Allodynia
 When damage occurs in tissues, pain fibers trigger the feeling of pain. The new information shows that new kinds of circuits are then either created through neuroplasticity, or they exist already as microcircuits that are normally always inhibited. The change that occurs can disinhibit these existing small circuits and produce unusual types of pain.
When damage occurs in tissues, pain fibers trigger the feeling of pain. The new information shows that new kinds of circuits are then either created through neuroplasticity, or they exist already as microcircuits that are normally always inhibited. The change that occurs can disinhibit these existing small circuits and produce unusual types of pain.
One unusual type of pain has long been known, but with no mechanism understood. This is called allodynia. Allodynia is when pain is sensitized to the point where stimuli that wouldn’t normally cause pain, such as light touch, produces significant pain. Also it can occur in new places. It is different from another unusual pain called hyperalgesia. Hyperalgesia is when something that normally causes mild pain causes a much greater pain. One unusual finding is that opiods can produce hyperalgesia.
Many of the new microcircuits are related to allodynia, which is surprisingly frequent. It occurs in as many as 50% of neuropathies (damage to nerves) and higher order brain signals for pain. We still don’t understand which sensory neuron is involved because there are many different nerve types that carry light touch.
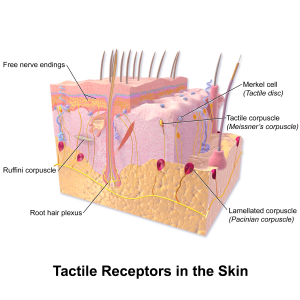 There is more known about mechanical touch receptors that do not cause allodynia. This involves the channel receptor called Piezo2 for cat ions (positive charge such as sodium and potassium). When these are eliminated in research, there is no light touch, but there is still mechanical pain of the normal variety. It also might be involved in allodynia.
There is more known about mechanical touch receptors that do not cause allodynia. This involves the channel receptor called Piezo2 for cat ions (positive charge such as sodium and potassium). When these are eliminated in research, there is no light touch, but there is still mechanical pain of the normal variety. It also might be involved in allodynia.
Now, allodynia appears to be related to altered circuits in the dorsal horn. Touch normally appears to stop pain sensation in dorsal horn circuits through a feed forward circuit. After injury, this mechanism is faulty when allodynia occurs. So that the feed forward inhibition is broken and light touch impacts on the pain circuit. A fiber responding to low threshold stimulation of mechanical touch signals to the lamina 1 of the dorsal horn. This triggers neurons that signal multiple other sources when this inhibition is not present. What is unusual about this research is that the allodynia appears to be a preexisting circuit that is triggered, not something unusual and or a newly created circuit.
Allodynia Circuit in Dorsal Horn
This circuit is more complex than previously thought. One part of it consists of excitatory interneurons that goes to a region between lamina 2 and 3. This produces a kinase enzyme C signal and goes to neurons in lamina 1 that then travel to many other regions of the cortex and emotional centers.
Recently, many new parts of this allocynia circuit were found. Two new parts of the circuit occur in layer two. One is called a “transient central cell”. The other vertical cells in outer layer 2. These both appear to be complex receiving a wide variety of different inputs. They seem to analyze many different sensory inputs. Another section of this circuit is in layer 3 from low threshold sensory neurons and it carries pain. Yet another part has neurons with neurotransmitter calretinin in inner layer 2.
Previously the dorsal horn in the spinal cord was considered to be only a relay point of pain fibers that could be altered by neurons from above. Now it appears there are many small circuits within the dorsal horn. Even more surprising is that these small micro circuits within the dorsal horn are different based on whether the original damage that precipitated pain is from breaks in neurons or from inflammation.
Gates for Allodynia Microcircuits
 The microcircuits mentioned above involve interneurons that are excitatory. It appears that the inhibitory interneurons in the dorsal horn are critical as gates that stop allodynia from occurring normally. In particular, stopping the Parvalbumin (PV) interneurons in layer 3 can produce allodynia related to kinase C mentioned above. PV interneurons can stop allodynia in research experiments after nerve injury. PV works in several different circuits with different sensory fibers.
The microcircuits mentioned above involve interneurons that are excitatory. It appears that the inhibitory interneurons in the dorsal horn are critical as gates that stop allodynia from occurring normally. In particular, stopping the Parvalbumin (PV) interneurons in layer 3 can produce allodynia related to kinase C mentioned above. PV interneurons can stop allodynia in research experiments after nerve injury. PV works in several different circuits with different sensory fibers.
Another interneuron with the neurotransmitter dynorphin (an endogenous morphine like neurotransmitter) in 2 and 3 can stop allodynia. This gate controlls interneurons with somatostatin neurotransmitter, which is a large group in the dorsal horn. They appear to work in sync with the kinase C and the vertical neurons.
There are multiple different small microcircuits related to this unusual form of pain and several different gates controlling these circuits.
Disinhibition
 The normal function of the dorsal horn is altered to produce allodynia. Also, central sensitization is related through the modulating circuits from the higher brain. There are several ways these alter the dorsal horn function to produce allodynia.
The normal function of the dorsal horn is altered to produce allodynia. Also, central sensitization is related through the modulating circuits from the higher brain. There are several ways these alter the dorsal horn function to produce allodynia.
One pathway involves injury stimulating BDNF (brain derived neurotrophic factor – a major factor that helps build neurons). BDNF alters potassium channels in a receptor called TRKB (tropomysin receptor kinase B). This stimulates pain fibers by stopping inhibition of allodynia circuits.
This mechanism is different in males, which activates microglia, and females, which activates T cells. Microglia are triggered by ATP in receptors PsX4 receptors, which send cytokines for microglia. The T cell needs to be worked out, but it is striking that males and females would have such different pathways.
Pain Circuits In the Brain
 There are major questions about how specific higher circuits produce the different types of pain. Are there specific small sub circuits in the cortex?
There are major questions about how specific higher circuits produce the different types of pain. Are there specific small sub circuits in the cortex?
Multiple higher brain regions are involved in pain from toxins and from internal organs. These “pain matrix regions” include circuits in the cortex—cingulate cortex (ACC), insular cortex, sensory cortex, and prefrontal cortex. Also active are thalamus, basal ganglia, cerebellum, and amygdala. It is not clear whether these are specific to pain or rather these centers work by emphasizing that it is a significant event to pay attention to.
Posterior insular cortex signals strength of pain stimulation—stimulation of this center causes pain. The insula is very significant for its multiple roles in integrating emotion, thinking, as well as pain in all its types. Sub regions relate to various senses—seeing, hearing, touching. Others relate to touch and to toxins. It is not only a pain center. Without a particular sodium channel (Nav1.7), people have little pain experience even with damage, but activity continues in the region.
 Studies point to the fact that small sub regions in all of the “pain matrix regions” are related to pain, not the larger regions. These micro circuits are too complex to be sorted out at this time. Some regions show possible specific circuits for social pain, direct physical pain, and feeling the pain of others.
Studies point to the fact that small sub regions in all of the “pain matrix regions” are related to pain, not the larger regions. These micro circuits are too complex to be sorted out at this time. Some regions show possible specific circuits for social pain, direct physical pain, and feeling the pain of others.
Other studies show that each region mentioned has multiple different functions, with pain being only one part. Specific forms of neuroplastic reinforcement of circuits is being found for the relation of anxiety and chronic pain in the ACC synapses.
The nucleus accumbens brain center relates to motivation (addiction) and also to subjective experience of pain. When pain is produced in animal experiments from damage to nerves or inflammation, there are alterations in the nucleus accumbens such as more release of galanin causing neuroplastic changes. These circuit alterations use a form of neuroplasticity called long term depression (less synaptic strength) in dopamine 2 receptors in medium spiny neurons (D2-MSN).
Blocking Newly Discovered Peripheral Circuits as Treatments
 In the fetus and adults nerve growth factor (NGF) stimulates the receptor TRKA in the peripheral neurons. This signal produces pain from injuries and many diseases—low back injury, bone cancer, neuron damage from diabetes, arthritis. Blocking NGF is a new possible treatment for pain (using antibodies called tanezumab). Blocking the receptors might help as well.
In the fetus and adults nerve growth factor (NGF) stimulates the receptor TRKA in the peripheral neurons. This signal produces pain from injuries and many diseases—low back injury, bone cancer, neuron damage from diabetes, arthritis. Blocking NGF is a new possible treatment for pain (using antibodies called tanezumab). Blocking the receptors might help as well.
Sodium channels in pain fibers are part of pain as well (channels Nav1.7 and Nav1.8). Inhibiting these helped experimental pain as much as morphine. Antibodies against these channel receptors also decreased allodynia.
Research uses animal venom from snakes to identify pain fibers. One toxin affects a particular channel (ASIC1) causes pain and blocking this receptor in the high brain regions did the opposite.
There are many other avenues of research via many complex pathways recently being discovered. For example capcaicin triggers the ion channel TRPV1 and blockers of that channel have stopped pain in mouse experiments of produced pain.
Genes have been introduced to alter abnormal production of proteins in these circuits to turn them back to normal. In one experiment, microRNA miR-7a is decreased with damage to peripheral neurons (neuropathic pain). Injections of this microRNA stopped allodynia and hyperalgesia after nerve injury. Nerve injury stimulates more of the sodium channel Nav1.3 and an RNA works against that with the result of stopping allodynia.
Many Unanswered Questions
There are many unanswered questions. It is still not known how mechanical stimulation occurs in sensory neurons. Many sensory neurons are not known. The coding of which neurons carry what types of pain are just being discovered. The micro circuits in the dorsal horn are just being found. And research has just scratched the surface of the many long projection neurons from the dorsal horn to parts of the higher brain regions. The many different ways pain occurs (inflammation, tissue damage, heat, cold, neuronal damage, etc) are not known. The central brain circuits for different kinds of chronic pain are not known.
Newly Discovered Circuits Produce Unusual Pain Syndromes
 New exciting research has found a wide variety of non-neuron cells are part of newly discovered signaling pathways that produce a variety of chronic pain syndromes. Recently, small micro circuits within the dorsal horn of the spinal cord have begun to be found that appear to begin to explain allodynia.
New exciting research has found a wide variety of non-neuron cells are part of newly discovered signaling pathways that produce a variety of chronic pain syndromes. Recently, small micro circuits within the dorsal horn of the spinal cord have begun to be found that appear to begin to explain allodynia.
The previous post shows signals from many different cells producing pain pathways are just being discovered and opens up a large number of new types of circuits and pathways. This includes astrocytes, microglia, cancer cells, tissue cells, lining cells, blood and immune cells.
This post shows early research into micro circuits in the spinal dorsal horn that can produce allodynia. These circuits are normally inhibited. Disinhibition of these circuits by interneuron gates is, at least, part of the explanation of allodynia. Hopefully this will lead to new treatments.