 In the search for eternal youth, it is not clear what causes cellular aging. It is, also, not known how much individual cells contribute to the aging of organs, tissues and animals. Recent research with individual cells shows that the process of cellular aging is not simple. There now appears to be many different possible causes and outcomes to a cell showing the signs of aging.
In the search for eternal youth, it is not clear what causes cellular aging. It is, also, not known how much individual cells contribute to the aging of organs, tissues and animals. Recent research with individual cells shows that the process of cellular aging is not simple. There now appears to be many different possible causes and outcomes to a cell showing the signs of aging.
Just as cells are able to repair damage to their own DNA and mitochondria, it is possible that cell’s can make choices to avoid aging. In fact, recently it has become clear that cells and tissues use cellular aging proactively for many different critical purposes. For example, in wound healing, cells deliberately age in order to avoid making a more severe scar. In the embryo and adult tissues, cells age as a mechanism to not overbuild. They, also, pre plan the tidy cleanup of the debris from the dead cell. The dynamic between necessary rapid cellular aging and chronic debilitating cellular aging is only now coming into focus. Just as cells are able to edit their own alternative messenger RNA, and fix breaks to their DNA, they are able to use many different pathways to make choices about individual cellular aging. Can individual cells choose not to age?
Many Forces and Pathways Contribute to Cellular Aging
 Aging of cells has been considered an irreversible process that protected the cell from turning into cancer. But, recent research shows a much more complex process, where the cell has many forces operating on it and some choices. There appears to be many different states that can develop after the ordinary growth stops.
Aging of cells has been considered an irreversible process that protected the cell from turning into cancer. But, recent research shows a much more complex process, where the cell has many forces operating on it and some choices. There appears to be many different states that can develop after the ordinary growth stops.
At some point cells stop dividing, which goes along with alterations in the chromatin, the metabolic processes that create secretions, and the efforts to avoid cancerous changes. The end of cellular division has been linked to a reduction in the length of telomeres (the tips of chromasomes), which is correlated with DNA instability and development of cancer. It was thought that as the division process becomes unstable, cancer could result and that this might be the way cells avoid cancer. Now, there appears to be many more factors that are important including the original fetal development, injuries, tissues that have been repaired and the aging of the entire organism. It, also, has been discovered that cells can grow their telomeres.
There are many other considerations including “assisted cell cycling,” multi step senescence, as well as acute and chronic and factors after division stops.
There Are Many Causes of Cellular Aging
This section tries to briefly outline the enormous complexity of aging pathways.

The significant cellular signaling pathway called the DNA damage response (DDR) appears to be connected to loss of telomeres, general DNA stress, DNA lesions and reactive oxygen. This complex process involves various kinases, stabilization of the factor p53 and the inhibitor p21. Other causes of cell aging are specific genes that promote cancer. These genes when activated cause incorrect copying of DNA, breaks in both DNA strands stimulation of the DDR signaling cascade.
But, there are other completely different causes of cellular aging that have nothing to do with these pathways. One involves mitochondria and their connection to the DDR pathway. Also, many studies show that aging involves great metabolic changes in the cell. Another pathway is the inhibition of tumor suppressor genes.
Cytokines can cause cellular aging such as with interferon β. Production of cytokines that trigger inflammation and chemokines attract immune cells that are associated with almost all of these pathways. Also, several of these pathways can work together.
There are, also, pathways that stimulate cell division that have nothing to do with cancer. There are epigenetic causes, as well as stress on the spindle, which directs cell division. Histone deacetylase inhibitors can trigger aging in a totally different way. Inhibition of the RNA polymerase inhibition triggers p53, which can cause cellular aging.
 Some aging cells respond to stress and can return to normal division. In others, cells have chronic stress that damages the cell. The cell will start to divide through special support pathways that are not usually used connected with p53 and p21. This type alters the metabolic pathways. This “assisted cell division” includes deterioration of some function and the start of cellular aging.
Some aging cells respond to stress and can return to normal division. In others, cells have chronic stress that damages the cell. The cell will start to divide through special support pathways that are not usually used connected with p53 and p21. This type alters the metabolic pathways. This “assisted cell division” includes deterioration of some function and the start of cellular aging.
In all of the pathways, ultimately p53 and p16 are involved as well as inflammatory cytokines. These three final pathways can work together or oppose each other. Also, there are some pathways that are reversible. In fact, aged cells by any definition have been recently turned into stem cells, which are essentially born again.
Also, cellular aging is not simple or one thing. It is a multi step process that evolves.
Multiple Steps in Cellular Aging
 Cellular aging is a very active multi step process that evolves and changes over time. The individual cell makes many choices. It begins when the division process stops through action of p21 and/or p1 and involves both genetic and epigenetic changes. All the factors together are called the senescence messaging secretome SMS, or more commonly, the senescence associated secretary phenotype (SASP).
Cellular aging is a very active multi step process that evolves and changes over time. The individual cell makes many choices. It begins when the division process stops through action of p21 and/or p1 and involves both genetic and epigenetic changes. All the factors together are called the senescence messaging secretome SMS, or more commonly, the senescence associated secretary phenotype (SASP).
There are some cells that are just quiet; some, including neurons, don’t divide because they are the end result of development; and there are cells aging through SASP.
SASP factors are critical to different kinds of aging. It includes multiple signals including cytokines, secreted proteins, inflammation, growth factors, and enzymes that alter other cells. Signaling can stimulate aberrations of mitochondria that produce too much reactive oxygen (ROS), which damages DNA and continues a damaging cycle.
The p16 and p53 pathways lead to early aging. Then, a large number of different SASP factors alter the chromatin and damage DNA. Some of the SASP factors stop cell division. Some of these factors lead to the cells being eaten by immune cells. Some microRNAs that are produced avoid this clearance.
 From this early aging, further deterioration is caused through breakup of the histones by enzymes and actions of jumping genes. It appears that aging cells contribute to local inflammation. The very late aging involves jumping genes transcribed as if they were genes, which creates chaos.
From this early aging, further deterioration is caused through breakup of the histones by enzymes and actions of jumping genes. It appears that aging cells contribute to local inflammation. The very late aging involves jumping genes transcribed as if they were genes, which creates chaos.
Another factor that increases aging is chromatin and DNA that is pushed from the nucleus into the cytoplasm. This unusual process is called chromosome budding or cytoplasm chromatin fragments (CCF), where histones are lost.
Acute and Chronic Cellular Aging
There are two distinct types of cellular aging—acute aging and chronic aging. Differences between them include the ways the cells function, the speed of function, and the SASP factors. Different types of cellular aging impacts wound healing, cancer and human aging.
Acute Cellular Aging
 Acute aging is highly regulated in wound healing, specific tissues and in embryos. Here the SASP factors are programmed and designed for a brief life of the cells that could otherwise live much longer. This occurs with one group of cells in a tissue only, not most of them. It is similar to apoptosis and uses parts of the apoptosis pathways. It targets specific types of cells. Clearance of the old dying cells is pre arranged.
Acute aging is highly regulated in wound healing, specific tissues and in embryos. Here the SASP factors are programmed and designed for a brief life of the cells that could otherwise live much longer. This occurs with one group of cells in a tissue only, not most of them. It is similar to apoptosis and uses parts of the apoptosis pathways. It targets specific types of cells. Clearance of the old dying cells is pre arranged.
Aging of cells occurs in wound healing when the work is completed and the many cells must die to stop more scarring and problems. Molecules in the extra cellular matrix stimulate this type of cellular aging. As these cells age, they undergo the SASP state producing ROS, which limits the development of scars.
 This, also, occurs in the repair of organs such as the liver and in embryo implantation for maternal blood. Acute cellular aging is planned and is a self-limited process. The clearance of the defective cells is well organized.
This, also, occurs in the repair of organs such as the liver and in embryo implantation for maternal blood. Acute cellular aging is planned and is a self-limited process. The clearance of the defective cells is well organized.
In other forms of cellular aging, many factors impinge on the cell and the temporary aspect is altered. Then, clearing of the aging cells doesn’t occur, because the immune system is negatively impacted.
Cellular aging during cancer treatment appears to have elements of both. The acute aging kills the cells; the chronic aging allows cells to be damaged but continue on.
Chronic Cellular Aging
 Chronic aging occurs after periods of intense stress to cellular functions. It can be stimulated by damage to the genetic functioning causing the cell to stop dividing. It is not pre organized and appears somewhat random. It occurs in only some cells of a tissue and is not like a disease that hits all of the members of one cell type. It is increased by a decreased immune system function, which occurs with organism aging. Signaling of inflammation can be decreased, which means that immune cells don’t respond as well. In cancer treatment, cells can have acute aging that turns into chronic. Clearance is not arranged and is random.
Chronic aging occurs after periods of intense stress to cellular functions. It can be stimulated by damage to the genetic functioning causing the cell to stop dividing. It is not pre organized and appears somewhat random. It occurs in only some cells of a tissue and is not like a disease that hits all of the members of one cell type. It is increased by a decreased immune system function, which occurs with organism aging. Signaling of inflammation can be decreased, which means that immune cells don’t respond as well. In cancer treatment, cells can have acute aging that turns into chronic. Clearance is not arranged and is random.
Neurons are fully differentiated terminal cells (although they can live for a hundred years), but they accumulate DNA damage over time. They have other elements of the aging process—cyotkines that cause inflammation, alterations of chromatin structures, and β-galactosidase activity. It is not clear whether neurons develop a full SASP that damages nearby cells.
Organ Deterioration
The chronic aging of specific cells can increase the general aging of the tissues of a body, and later the entire body. Cellular aging decreases the amount of necessary cells because they stop multiplying. This interferes with the normal maintenance of the tissue or organ. It decreases the normal ability of an organ to regenerate. When SASP starts it can cause all kinds of havoc including inflammation.
 Aging cells damage the local regions where stem cells live. Stem cells are critical for the function of the tissue by replacing cells. (The stem cell niche was described in the posts on The Very Intelligent Intestinal Epithelial Cell, and The Intelligent Skin Cell). Aging cells produce many different secretions, including enzymes that disrupt the local tissue structure. Specific cytokines that are secreted IL-6 and IL-8 create scars in many tissues.
Aging cells damage the local regions where stem cells live. Stem cells are critical for the function of the tissue by replacing cells. (The stem cell niche was described in the posts on The Very Intelligent Intestinal Epithelial Cell, and The Intelligent Skin Cell). Aging cells produce many different secretions, including enzymes that disrupt the local tissue structure. Specific cytokines that are secreted IL-6 and IL-8 create scars in many tissues.- Aging cell damage disrupts the critical extra cellular matrix (see post on Extracellular Matrix in Neuroplasticity.)
- When new cells become faulty, they impair the structure of the tissue. The many secreted factors of SASP create an environment that stimulates cancer in some tissues. Cancers dramatically increase as the creature ages.
 The aging process stimulates a dangerous form of inflammation. It attracts many lymphocytes and macrophages (cells that can eat debris and other cells – like microglia). This causes the aging of tissues and specific cell death. There are many other cytokines that appear to be related to inflammation that creates aging damaged tissue.
The aging process stimulates a dangerous form of inflammation. It attracts many lymphocytes and macrophages (cells that can eat debris and other cells – like microglia). This causes the aging of tissues and specific cell death. There are many other cytokines that appear to be related to inflammation that creates aging damaged tissue.- One aging cell can cause neighboring cells to become aged through secretions of factors, including cytokines.
Aging cells are found in many major diseases including Alzheimer’s, osteoarthritis, and diseases caused by clogged arteries.
It has been difficult to study aging pathways because p53 and p16 are so powerful that they are involved in many other processes. They are critical for cellular reproduction, cancers, and cell death.
But, it does appear that the more individual aging cells there are, the more there is general aging. It is not clear exactly how the tissue dysfunction occurs.
Major Aging Pathways
 Many different events can trigger the pathway that leads to aging. Various stressors can trigger general pathways that have many possible outcomes. These stressors include triggering genes that cause cancer, and losing genes that suppress cancers, damage to the telomere, stress from oxidative metabolic toxins (ROS), damage to DNA, damage to the histones, damage to the spindle for cell division, and other factors.
Many different events can trigger the pathway that leads to aging. Various stressors can trigger general pathways that have many possible outcomes. These stressors include triggering genes that cause cancer, and losing genes that suppress cancers, damage to the telomere, stress from oxidative metabolic toxins (ROS), damage to DNA, damage to the histones, damage to the spindle for cell division, and other factors.
Ultimately all of these cascades trigger p53, and p16 or both. The post on the inverse relationship of cancer and degenerative brain disease described these critical pathways as a continuum from generation of new cells to deterioration of old cells. The p53 pathway is a critical cascade in both. In one direction of growth, p53 can lead to making new healthy cells or in the extreme making cancerous cells. In the other direction, p53 can lead to degeneration of cells causing degenerative brain disease. In this way cancer and dementia are opposites.
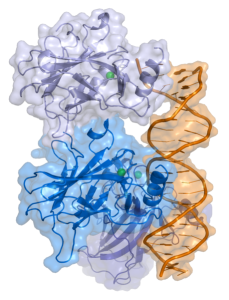
A special factor p21 is sometimes triggered in the p53 pathway that involves stopping cell reproduction. This stoppage can be temporary or permanent.
There are two temporary situations, one where the damage is repaired, and another where aid is given to a damaged cell that continues to divide. The pathways that support reproduction occur when there is moderate chronic stress to the cell—assisted cell cycling. There are different cycles that help or hurt this process.
There are other stressors and pathways related to metabolic cycles that create ROS. There is always the possibility of inflammation, caused by the stress or a result of the stress. Even with severe stress there are multiple pathways to reverse it, or work with it. The cells are very well positioned to reverse or avoid it.
One study showed that when old stem cells are exposed to young new stem cells, the old cells become stronger and more able to make new cells. This, also, occurs when they are placed in young tissue.
Why Do Aging Cells Accumulate?
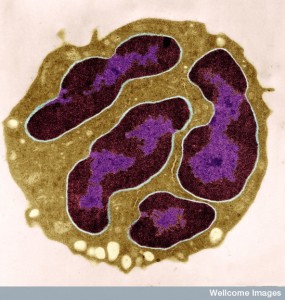 Multiple stresses lead to the gradual aging of specific cells. This process can take a very long time. But, also, the clearance mechanisms can become faulty. Often, immune cells eliminate faulty cells. Individual cells might trigger signals that they are faulty and attract immune cells to remove them.
Multiple stresses lead to the gradual aging of specific cells. This process can take a very long time. But, also, the clearance mechanisms can become faulty. Often, immune cells eliminate faulty cells. Individual cells might trigger signals that they are faulty and attract immune cells to remove them.
These immune processes for self-elimination are similar to self-destruction pathways. For this, many different immune cells are attracted by cytokines—T cells, macrophages, and natural killer cells. If the immune system as a whole is affected by the aging process, the signals for self-elimination are decreased.
Acute aging of cells is highly organized and planned. Chronic aging involves many different factors, and triggers a large number of signals that have varied effects. Some of these factors create cells that are resistant to clearance by immune cells and create further tissue deterioration.
Telomerase Switch
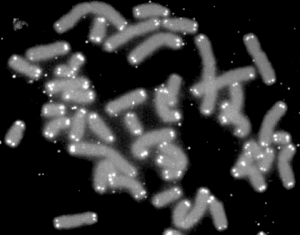 While the deterioration of telomeres was viewed, until recently, as immutable, new research shows a switch that allows it to rebuild. In the normal process of cell division, some of the telomere DNA is lost. The enzyme telomerase carries its RNA template to put back some of the lost material making the telomere longer, but not quite as long as before.
While the deterioration of telomeres was viewed, until recently, as immutable, new research shows a switch that allows it to rebuild. In the normal process of cell division, some of the telomere DNA is lost. The enzyme telomerase carries its RNA template to put back some of the lost material making the telomere longer, but not quite as long as before.
In many bodily organs, as cells deteriorate other cells continue to divide to replenish the organ. But, there appears to be a limit to the number of divisions, correlated with telomeres at the end of the chromosomes shortening. When the telomere is too short, the cell can’t divide anymore.
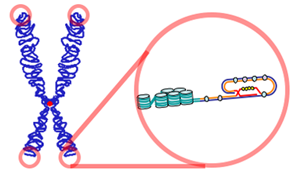 Some cells, however, produce more telomerase, which rebuilds the telomere allowing more divisions. New studies show that telomerase can be switched on and off. When off it may break up into parts, so even if it is present, it may not be active.
Some cells, however, produce more telomerase, which rebuilds the telomere allowing more divisions. New studies show that telomerase can be switched on and off. When off it may break up into parts, so even if it is present, it may not be active.
In a complex process that is not well understood, during cell division, after the genes are fully copied, a missing part re assembles the telomerase. However, once it is assembled it then disassembles, switching it off. In cancer cells, there is more telomerase and the cells don’t stop dividing.
Also, telomeres have very recently been shown to do more than determine when the cell doesn’t divide. The telomeres interact through loops with specific loci on the DNA and as they become shorter, they alter which DNA they are activating. By rebuilding the telomere, cells can stimulate the previously used regions of DNA.
This research implies that it is possible to switch the telomerase back on to rebuild the telomere and avoid the demise of self replication.
Clearing Away Cellular Debris
Recent studies show that increased clearance of defective cells can greatly decrease degenerative diseases. In a previous post it was demonstrated that misfolded proteins and debris from the brain are cleaned out in various ways.
The two major ways are the newly discovered “glia – lymphatic” or glymphatic system, and the normal function of microglia that eat debris. Both mechanisms remove amyloid that accumulates in Alzheimer’s. Also, elimination of defective cells and debris can be greatly enhanced by lifestyle changes (see post on Five Secrets of Brain Health.)
An experiment showed that stimulating pathways that clear away debris decreases the diseases that are caused by additive cellular aging. This process didn’t show adverse effects. Some of the techniques that have been developed for clearing cancer cells are similar to ones that could eliminate the aging cells that will cause future trouble. In fact, it is easier because if some cells remain, they don’t reproduce, whereas if cancer cells are left, they do start building the cancer again.
We still don’t know how aging cells stimulate aging of the entire organism. Another problem is that each aging cell has unique SASP factors among the many possible. Other critical factors are:
- Telomere reduction as a stressor is unique to humans and therefore is very difficult to study.
- Removal of all the aging cells could well hurt the function of the tissues.
- Immunodeficiency, by itself, doesn’t greatly increase aging individual cells.
Can Individual Cells Choose Not to Age
 Just as cells edit their own DNA and alternative RNA splicing, they, determine programmed cell death and acute cellular aging. In the acute cellular aging process, cells specifically program their own death and the elaborate pre programmed cleanup. This occurs in wound healing to avoid scars, in the embryo and in adult organs to avoid overbuilding.
Just as cells edit their own DNA and alternative RNA splicing, they, determine programmed cell death and acute cellular aging. In the acute cellular aging process, cells specifically program their own death and the elaborate pre programmed cleanup. This occurs in wound healing to avoid scars, in the embryo and in adult organs to avoid overbuilding.
Cellular aging is an extremely complex process that involves a large number of different pathways. While some of the factors that trigger these pathways are responses to environmental events, there is definite evidence that cells make choices along the way. It does appear that individual cells can in some ways determine whether they will die or live a very long life.
Where is the direction for individual cells not to age in order to help avoid a scar or overpopulation of an organ? Where is the direction for the many cellular choices? Where is the direction for a cell editing its own DNA and RNA. Does the intelligence of cells come from contact with forms of mind throughout nature?

