 Human cells are massively larger and more complex than bacteria and yet microbes keep up relentless intelligent warfare. Previous posts documented surprisingly sophisticated, multi level attacks by microbes using protein molecules and micro RNA against plants and animals.
Human cells are massively larger and more complex than bacteria and yet microbes keep up relentless intelligent warfare. Previous posts documented surprisingly sophisticated, multi level attacks by microbes using protein molecules and micro RNA against plants and animals.
Recently, new microbe techniques have been discovered that specifically target organelles of the human cell. These complex assaults can directly manipulate genes to produce unique proteins. These altered proteins change normal functions to produce new types of activity helpful to the microbe. This activity, somehow, occurs in complex pathways with cascades of interacting proteins. Microbes, also, use epigenetic tags on DNA and histones. In order to use epigenetic techniques, microbes are able to target specific enzymes that alter the function of genes through the histones and other structures protecting them. These assaults alter the gene expression by placing specific tags blocking the functions of regions of DNA. This is done either indirectly by altering the normal enzymes that perform these functions, or directly by placing and removing the tags with their own produced molecules.
 To perform these sophisticated manipulations, many levels of interacting codes are utilized. It is hard to imagine how unicellular microbes can understand the effect that a tag will have on the underlying gene that will alter the specific amino acid sequence to alter the exact shape of a protein. This particular protein shape, then, has very particular functions in complex cascades in the human cell. Also, these molecules are injected into the cell by extremely complex secretion systems that look similar to a syringe. This is highly intelligent activity for a small single celled creature.
To perform these sophisticated manipulations, many levels of interacting codes are utilized. It is hard to imagine how unicellular microbes can understand the effect that a tag will have on the underlying gene that will alter the specific amino acid sequence to alter the exact shape of a protein. This particular protein shape, then, has very particular functions in complex cascades in the human cell. Also, these molecules are injected into the cell by extremely complex secretion systems that look similar to a syringe. This is highly intelligent activity for a small single celled creature.
Despite extremely complex functions in the cell’s nucleus, mitochondria, endoplasmic reticulum and Golgi, these very small intelligent microbes are able to manipulate and commandeer organelles for their own advantage. Attacks are extremely precise and allow the microbe to hide inside the cell compartments to reproduce by disabling surveillance and immune responses. Throughout evolution, both eukaryote cells and microbes developed methods of attack and cellular level immune defenses. This post will describe how intelligent microbes attack organelles.
Organelles Have Particular Territories and Functions
 The eukaryote cell is very organized into compartments. These discrete regions of the cell have specific functions. Microbes manipulate the organelle’s unique functions to gain advantage over the cell.
The eukaryote cell is very organized into compartments. These discrete regions of the cell have specific functions. Microbes manipulate the organelle’s unique functions to gain advantage over the cell.
Mitochondria produce energy and manage programmed cell death (apoptosis); the nucleus maintains and regulates the complex use of DNA and RNA; the endoplasmic reticulum (ER) handles and sorts new proteins and lipids; the Golgi directs secretory pathways where materials are carried to every section of the cell in vesicles.
Microbes have developed specific proteins that are secreted to attack each of the organelles—called effector proteins—which alter the cell’s functions. The secretion of these proteins is through one of 7 different elaborate secretion systems, but especially types 3 and 4 are utilized in this battle between cell’s organelles and bacteria. Microbes are able to direct secretions precisely and at the exact time that can be most effective.
One of the microbe strategies is to make proteins that are very similar to the cell, but that alter function in various ways. They develop these either through evolution in the midst of battle with the cell or by acquiring the genes.
An example occurs with the amoeba, which makes proteins that are very close to those usually only found in animals and secretes them into the cell. Legionella sends more than 300 different proteins into a human cell. These proteins are otherwise only found in human cells. Other bacteria have been found with the same exact genes by horizontal gene transfer among many microbes. Some microbes, such as Chlamydia and Legionella produce proteins with sections that are similar to a variety of different human proteins and have multiple effects.
A previous post described the ubiquitin system of labeling molecules in the cell, which has thousands of variations. The cell designates functions and destinations for molecules by using this very complex tagging system. Microbes have picked this up and use this same tagging system in warfare with cells to counteract functions. See the post describing intelligent warfare using ubiquitin and another labeling system called sumolyation.
Targeting an Organelle

In the normal function of the cell, tags are placed on newly produced proteins and other molecules to show what organelle will be receiving them. Bacteria copy this approach and secrete signals via the type 3 secretion system (T3SS) for the nucleus (NLS or nucleus localization signal) and the mitochondria (MLS or mitochondrial localization signal). The type 4 secretion system (T4SS) can inject DNA. Injection of proteins and genetic material can occur from outside of the cell by E coli and this goes with a tag directly to the mitochondria. Other intracellular bacteria inject both outside and then inside the cell. Salmonella has multiple proteins injected and Legionella has 300 different proteins with different functions.
Another technique is delivery of toxins without secretion systems either outside or inside the cell. Listeria has a toxin that cuts a hole in the cell membrane and internal membranes.
Targeting the Cell Nucleus
 DNA is wrapped twice around histone protein for protection and must be unspooled when used. Molecular tails on the histones can regulate whether the spool will be opened to allow particular genes to operate, or not. These tails are important in regulating enzymes that place a wide variety of epigenetic tags on the histone proteins. There are now known to be more than fifty different kinds of tags and placements on the protein, such as methylation, acetylation and ubiquitination on a lysine amino acid and the phosphorylation on serine and threonine. These tags are generally called post-translational modifications (PTMs). These have major effects on regulating the operation of genes.
DNA is wrapped twice around histone protein for protection and must be unspooled when used. Molecular tails on the histones can regulate whether the spool will be opened to allow particular genes to operate, or not. These tails are important in regulating enzymes that place a wide variety of epigenetic tags on the histone proteins. There are now known to be more than fifty different kinds of tags and placements on the protein, such as methylation, acetylation and ubiquitination on a lysine amino acid and the phosphorylation on serine and threonine. These tags are generally called post-translational modifications (PTMs). These have major effects on regulating the operation of genes.
While the effects of each type of PTM is very complex and just being discovered, somehow microbes already understand them and hijack this system, particularly related to the cell’s response to identifying the microbe in the cell. The cell has a pattern recognition system to identify microbes called pathogen-associated molecular patterns (PAMPs) that stimulate immune pathways with cytokines. The microbes attack both the cascades that stimulate inflammation and, also, the histone tags.
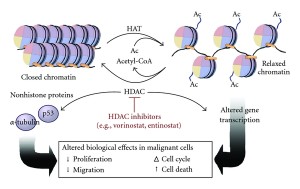
Major microbes, such as mycobacterium tuberculosis, influence the enzyme for acetylation (histone deacetylase or HDAC). They directly influence the gene that makes this enzyme. This is a remarkably complex mechanism for a microbe to utilize. Another microbe, A phagocytophilum, stimulates two genes that make two different HDACs causing less acetyls on histone 3. This decreases the function of two critical genes for the cell’s defense.
Some microbes go after the larger 3D structure of the DNA in the nucleus. Recent research (see previous post on genetic new genetic complexity) has shown that regulation of DNA is fantastically complex and now includes three-dimensional structures that hold the DNA in various positions inside the nucleus. (Manipulation of this structure by microbes is an entirely new field called patho-epigenetics.) Somehow, microbes understand much more than we do about how DNA is regulated. First the effector protein from the microbe has to get to the nucleus through special tags that take the molecule in through the very complex nuclear pore mechanism.
Multiple microbes, including E. coli, Shigella and Listeria, make proteins that alter the proteins that hold the DNA in place. First they influence the actin structures and then alter histones through special complexes of proteins. Listeria nuclear targeted protein A (LntA) binds to chromatin and stops critical genes that make interferon (IFN). IFN is a critical signal for general immune response.
There are now known to be multiple factors of this type that target the histone and alter their structure, silencing genes. Some of them interfere with extremely complex molecular cascades. One type silences the signals for apoptosis, a cell death signal, which is the ultimate response to a badly infected cell. This leads to lengthy infections.
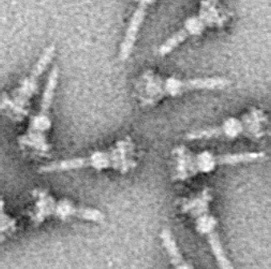 The microbe that causes the fatal disease dysentery secretes an effector with T3SS. It affects the phosphorus tag by interfering with the enzyme and alters the number of phosphorus tags on histone 3. This blocks another critical cytokine (NF-κB), which is a signal for inflammation. This same toxin has other elaborate effects on the production of critical molecules in innate immunity.
The microbe that causes the fatal disease dysentery secretes an effector with T3SS. It affects the phosphorus tag by interfering with the enzyme and alters the number of phosphorus tags on histone 3. This blocks another critical cytokine (NF-κB), which is a signal for inflammation. This same toxin has other elaborate effects on the production of critical molecules in innate immunity.
E coli causes severe diarrhea disease with a similar mechanism through secretion proteins through T3SS. It affects the enzyme that tags a histone with acetyls, called acetytransferase or HAT, and and this lowers its activity. This is another way to negate NF-κB. It, also, affects the vital p53 and interleukin-8 (IL-8)
Direct Attack on Chromatin Structures
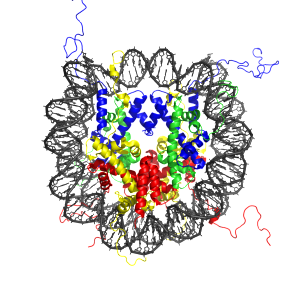
Chlamydia alters histones in a different way. Methylation of histones is a major way DNA is regulated either increasing or decreasing the effect of the local genes. This microbe makes a protein that, by itself, alters the tags replacing the effects of the human enzymes.
There are other versions of this technique used by pneumophila, which directly places methyl groups to replace an acetyl and dramatically decreases some gene activity. These affected genes are, also, vital for the production of molecules for the immune system. Another technique places a methyl group on the DNA that makes ribosomes, making more ribosomes that the microbe can use to make its own DNA. makes a toxin that places methyl groups on 8 places of histone1. This stops the production of materials for inflammation.
Yet, another microbe uses a different mechanism. It makes a proteins ankyrin repeat protein (AnkA) that is secreted by T4SS, goes to the nucleus and accumulates there. It binds directly to DNA at a particular promoter that makes particular peptides used by the immune system. Recently, many different microbes have been discovered with genes for AnkA.
Microbes Attack the Endoplasmic Reticulum (ER) and Golgi
 The very complex secretory pathway was described in a previous post. This pathway related to the ER and Golgi provides very complex lipids in combination with proteins for many structures in the cell, including all membranes. It is the ER that tags the new proteins for their destinations and starts them on this secretory pathway including lysosomes and endosomes. These molecules are placed in particular tagged vesicles that bud from the ER membranes and then in the Golgi they are modified, often with the addition of sugars. The Golgi then sorts them again for transport to all parts of the cell in vesicles.
The very complex secretory pathway was described in a previous post. This pathway related to the ER and Golgi provides very complex lipids in combination with proteins for many structures in the cell, including all membranes. It is the ER that tags the new proteins for their destinations and starts them on this secretory pathway including lysosomes and endosomes. These molecules are placed in particular tagged vesicles that bud from the ER membranes and then in the Golgi they are modified, often with the addition of sugars. The Golgi then sorts them again for transport to all parts of the cell in vesicles.
Special molecules serve as switches, through a complex set of enzymes and factors, and form large complexes that help direct this process of tagged transport. Using these switches, there are special transports of these vesicles from the ER surface (anterograde trafficking) and the other direction from the membrane to the ER (retrograde Golgi-ER transport).
There are other factors in regulating this very complex transport process to all parts of the cell. One involves seven different special complex lipids (phosphoinositide lipids) that occur in various compartments of the cell. One type is at the cell’s membrane; another is in the Golgi. These are coordinated through the special transport enzymes.
Microbes Attack the ER
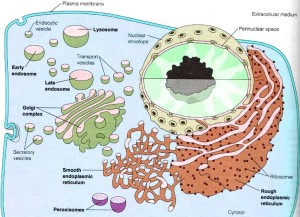 Microbes that attack the secretory system make a home in a vacuole inside the cell. Please see the previous post about this clever set of microbes that live in vesicles that often is used to destroy microbes by altering it. Legionella alters a vacuole by interacting with the ER vesicles (Legionella-containing vacuole or LCV). The LCV fuses with ER vesicles.
Microbes that attack the secretory system make a home in a vacuole inside the cell. Please see the previous post about this clever set of microbes that live in vesicles that often is used to destroy microbes by altering it. Legionella alters a vacuole by interacting with the ER vesicles (Legionella-containing vacuole or LCV). The LCV fuses with ER vesicles.
Legionella uses many injected molecules to accomplish this. They use the special lipids for their own purposes by manipulating the switches that control the secretory system. They accomplish this in unusual ways, such as trapping important molecules in the vacuole and then altering their functions by the attachment of tags. They alter the process where the ER makes the vesicles in the secretory pathway through very complex mechanisms. They make specific enzymes that sit on the membrane of the vacuole.
Chlamydia hijacks the Golgi vesicles. Salmonella, also, lives in a vesicle (Salmonella-containing vacuole SCV) in the Golgi. Many others do versions of this.
 Other microbes influence other vesicles, those between the ER and the Golgi. Pneumophilia targets this pathway along with the retrograde pathway. It makes proteins that inhibit the functions of the pathways. It manipulates the entire pathways to hide in a vacuole. Their vacuole is remarkable in that it has many unique lipids that should be identified by the cell. But, they produce many proteins (more than ten) that alter the switches regulating the traffic. This microbe demonstrates an extremely sophisticated interplay with the human cell.
Other microbes influence other vesicles, those between the ER and the Golgi. Pneumophilia targets this pathway along with the retrograde pathway. It makes proteins that inhibit the functions of the pathways. It manipulates the entire pathways to hide in a vacuole. Their vacuole is remarkable in that it has many unique lipids that should be identified by the cell. But, they produce many proteins (more than ten) that alter the switches regulating the traffic. This microbe demonstrates an extremely sophisticated interplay with the human cell.
Brucella uses another mechanism. They hide in a vesicle from the ER (Brucella containing vacuole or BCV). This vacuole interacts with other lysosomes making a more acid environment, which stimulates the special secretory 4 system that secretes many effectors. This alters the BCV to become just like a regular ER vesicle that replicates itself. They accomplish this by altering the switches at the ER exit sites. They alter the secretory pathway, especially the retrograde pathway.
Attacking the Golgi
 Chlamydia forms its own special vacuole, called an inclusion, at the Golgi using T3SS. It doesn’t fuse with the vesicles but captures a vesicle and then causes damage in the Golgi that releases a large amount of lipids. Many of these lipids are placed on the inclusion. It alters the lipid metabolism and gathers many proteins into the inclusion that helps it reproduce. It specially stops the apoptosis pathways.
Chlamydia forms its own special vacuole, called an inclusion, at the Golgi using T3SS. It doesn’t fuse with the vesicles but captures a vesicle and then causes damage in the Golgi that releases a large amount of lipids. Many of these lipids are placed on the inclusion. It alters the lipid metabolism and gathers many proteins into the inclusion that helps it reproduce. It specially stops the apoptosis pathways.
Attacking Mitochondria
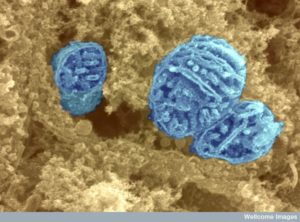 Mitochondria are semiautonomous microbes living symbiotically in every eukaryote cell. They have elaborate communication with the cell in many ways, but especially by docking at the ER. (See post on ER mitochondria communication in the neuron). They have two lipid membrane layers and have their own DNA that makes 12 very complex proteins for cellular respiration—the oxidative phosphorylation machines. Originally they made many more proteins but gave up these functions to the cell to focus on their main job of making energy through high-energy phosphate particles.
Mitochondria are semiautonomous microbes living symbiotically in every eukaryote cell. They have elaborate communication with the cell in many ways, but especially by docking at the ER. (See post on ER mitochondria communication in the neuron). They have two lipid membrane layers and have their own DNA that makes 12 very complex proteins for cellular respiration—the oxidative phosphorylation machines. Originally they made many more proteins but gave up these functions to the cell to focus on their main job of making energy through high-energy phosphate particles.
Mitochondria travel in the cell to provide energy where it is needed through elaborate signaling. In the neuron, large numbers use the axon transit system on microtubules (see the post on mitochondria travel in the neuron and transport along the axon). Mitochondria have an elaborate life cycle including fusing with others and breaking apart to make new ones. Their shape is altered by their functions—round and small for transport and long for many components. They have critical functions involved in maintaining calcium levels; manufacture of materials for proteins, lipids and nucleotides; and regulation of apoptosis or cell death.
 Recent research shows that they are critical for signaling in the fight with microbes including viruses
Recent research shows that they are critical for signaling in the fight with microbes including viruses
There are special proteins that have both signal tags for the nucleus and the mitochondria. They are not finished but exist in unfolded states with chaperones that can help them fold at a moment’s notice. The receptors notice these proteins and take them in to the mitochondria through pores. They are cut, folded and sent to very particular locations in the mitochondrial machinery sub compartment.
Microbes, such as tuberculosis and E. coli, use these same tags that send it to the mitochondria. This is a new area of research and not much is known about the tagging for the mitochondria. E. coli makes two effectors that go to the mitochondria by this described mechanism. One type decreases the function of the energy making and doesn’t affect apoptosis. The other type triggers apoptosis in the intestine and makes holes in the intestinal barrier.
Pneumophila makes a toxin secreted by T4SS that enters macrophages and attacks the mitochondrial second membrane. They form pores in the membrane that allow in too many metabolites, such as the high-energy particles to dissipate from the mitochondria.
 Cholera uses a T3SS secretion to send an effector to the mitochondria where it alters calcium flow, stops mitochondria from clumping together near the nucleus and affects the response to viruses.
Cholera uses a T3SS secretion to send an effector to the mitochondria where it alters calcium flow, stops mitochondria from clumping together near the nucleus and affects the response to viruses.
Another microbe inhibits apoptosis with a T4SS injected toxin, attacking mitochondria in white blood cells. The mitochondria cuts it, allowing it to travel into the center of the mitochondria through five membranes to the critical matrix. There it alters the critical cytochrome c mechanism and slows apoptosis. Another attacks autophagy mechanisms by sending toxins to autophagosomes.
Another toxin is beta-barrel proteins transported into mitochondria and placed in the membranes. They stop the energy process and kill the cell. Porins are, also, imported into mitochondria making holes in the membranes affecting cell death.
Mitochondria Control Cell Death (Apoptosis)
 Alpha-proteo-bacteria were the ancestors of mitochondria 1.5 billion years ago. Despite giving up many functions to the larger eukaryote cell, they kept the molecules for cell death. The most common attack on mitochondria relate to stopping programmed cell death or apoptosis. Mitochondria are uniquely involved in the decision whether or not to use this final response of the cell to being hopelessly infected. Microbes mostly often want to keep the cell alive in order to use its machinery and to stop apoptosis.
Alpha-proteo-bacteria were the ancestors of mitochondria 1.5 billion years ago. Despite giving up many functions to the larger eukaryote cell, they kept the molecules for cell death. The most common attack on mitochondria relate to stopping programmed cell death or apoptosis. Mitochondria are uniquely involved in the decision whether or not to use this final response of the cell to being hopelessly infected. Microbes mostly often want to keep the cell alive in order to use its machinery and to stop apoptosis.
The individual cell commits suicide to aid the larger organism in killing off dangerous microbes that have created a home in the cell. 60 billion human cells die each day. Apoptosis is a form of death that is pre programmed and in taking apart the cell, it doesn’t create inflammation and a scar as other types of cell death would. The cell shrinks, including the nucleus, where DNA is broken into pieces and the membrane forms blebs. The enzymes that perform these functions are caspases, which cut more than 600 different molecules into pieces including other caspase enzymes.
The apoptosis process starts when a large multi protein complex is assembled—the apoptosome—triggered by a protein from the mitochondria (cytochrome c) and one from the cell (APAF1 or apoptotic protease-activating protein). Cytochrome, normally, is part of the respiratory cycle and moves electrons. When it is sent into the cytoplasm it joins to trigger apoptosis. There are, in fact, several other molecules from the mitochondria that can do this and these all come through pores in the mitochondria membrane. The well-known protein BCL-2 that is linked with protection from cancer controls these pores. Many factors can trigger this process including damage to DNA with defective repair, toxic medications, radiation and other signals. Microbes have many toxins that affect this process in both directions.
Intelligent Microbes Attack Organelles
 To specifically attack the fundamental organelles of the cell, microbes use extremely sophisticated techniques that involve multiple levels of DNA codes, manipulation of histone codes, an understanding of how proteins are folded, and how newly produced versions of these proteins will interact in vastly complex pathways and cascades in human cells. They, also, build incredibly complex secretion weapons to send these molecules to particular places in the cell. Much of this is extremely difficult for humans to decipher and is currently unknown.
To specifically attack the fundamental organelles of the cell, microbes use extremely sophisticated techniques that involve multiple levels of DNA codes, manipulation of histone codes, an understanding of how proteins are folded, and how newly produced versions of these proteins will interact in vastly complex pathways and cascades in human cells. They, also, build incredibly complex secretion weapons to send these molecules to particular places in the cell. Much of this is extremely difficult for humans to decipher and is currently unknown.
How do small microbes, a thousandth the size of a human cell, understand how to do this? Meanwhile, our cells must understand the extremely varied attacks of a large number of different microbes and counteract them.
How can anyone not see the intelligent behavior in these microbes and in our cells?