 Hundreds of trillions of microbes live in the human gut, with 300 times the total DNA as humans. The products of this “forgotten organ” include large amounts of DNA that are critical to create necessary human nutrients, for essential metabolism and to develop the most effective immune system. Recent research shows dramatic effects of microbe products from the gut on mental function—depression, stress, autism, and degenerative illness. In humans, many studies show microbes affect anxiety, mood, depression and social behavior. Direct effects are through secreted products, stimulation of the enteric nervous system and travel of microbes into the brain, while indirect factors are microbes’ influence on immune function affecting behavior. Microbes produce molecules that transform into hormones and neurotransmitters or they produce neurotransmitters themselves. Microbes effect on the brain includes fetal development and neurotransmitter function.
Hundreds of trillions of microbes live in the human gut, with 300 times the total DNA as humans. The products of this “forgotten organ” include large amounts of DNA that are critical to create necessary human nutrients, for essential metabolism and to develop the most effective immune system. Recent research shows dramatic effects of microbe products from the gut on mental function—depression, stress, autism, and degenerative illness. In humans, many studies show microbes affect anxiety, mood, depression and social behavior. Direct effects are through secreted products, stimulation of the enteric nervous system and travel of microbes into the brain, while indirect factors are microbes’ influence on immune function affecting behavior. Microbes produce molecules that transform into hormones and neurotransmitters or they produce neurotransmitters themselves. Microbes effect on the brain includes fetal development and neurotransmitter function.
Microbes interact with immune cells, helping to form the immune system. Previous posts have shown that the intestinal epithelial cell and the skin cell are in constant back and forth communication with friendly and unfriendly microbes and the immune system backup if it gets out of hand.
Research increasingly shows pathways from the human gut to the brain influenced by microbes. There are many examples of particular parasites altering their hosts’ behaviour to benefit the microbe. But, also, trillions of microbes have life long relationships with normal humans. Many brain diseases co-exist with gastrointestinal problems such as autism, schizophrenia, depression and other degenerative brain diseases. One study showed that a metabolite from gut bacteria, alone, causes behavioural abnormalities of autism and anxiety. If you suffer with anxiety and have done for many years, you may want to check out the best weed strains for anxiety. Already in the USA, it has had great results from the few who have trialled the new treatment. Alternatively, an emerging new treatment is CBD oil. CBD is extracted from cannabis and has shown great promise in treating the symptoms of anxiety. It does not contain THC, the illegal element in marijuana, so it does not give a “high” when taken. You can buy CBD oil over the counter or online at places like www.cbdvillage.co.uk.
Mammals and microbes have had an intricate symbiosis for millions of years. Recently, the details of the relationship of microbes to our nervous and immune systems have been discovered. Microbes fight autoimmune diseases, boost the immune system, help maintain proper weight and decrease effects of stress. Microbes on the skin protect through stimulating immune function. Gut microbes can alter genes in the brain, which could account for some of the variations in medication effects.
 Microbes in the gut that don’t eat oxygen are hard to study because they can’t be cultivated. But, they are known to be important for the developing fetus, immune system, absorption of nutrients and gut motility. Diet has a major effect on the composition of the microbes. For example, Bacteroides occur with high fat or protein, whereas the Prevotella are associated with high-carbohydrate. The core microbes in the elderly are different from younger adults, and its composition is directly correlated with health.
Microbes in the gut that don’t eat oxygen are hard to study because they can’t be cultivated. But, they are known to be important for the developing fetus, immune system, absorption of nutrients and gut motility. Diet has a major effect on the composition of the microbes. For example, Bacteroides occur with high fat or protein, whereas the Prevotella are associated with high-carbohydrate. The core microbes in the elderly are different from younger adults, and its composition is directly correlated with health.
Microbes Affect the Behavior of their Host for their Advantage
Microbes are able to enter into the blood stream by affecting the cells lining the vessels. They can also enter red and white blood cells and are carried to neurons. Microbes can enter cerebrospinal fluid by affecting lining cells to make holes. They are able to enter peripheral nerves to travel along axons into the brain (see post on Varicella Virus) and they also enter through the olfactory system that is more exposed to the outside world than other brain regions.
In the brain microbes can stimulate many different illnesses and can change the behavior of the host, often to their own advantage.
- Aggressive biting behavior in dogs is caused by the rabies virus, which helps to spread the virus to other animals.
- Toxoplasmosis Gondi infection stimulates mice to be attracted to cats, which rapidly leads to their death and increases the ability of the microbe to infect another animal.

From Jamain The fungus Cordyceps infects an insect’s brain stimulating the insect to climb to the top of a plant, where the fungus destroys the insect’s brain by growing a fungus bloom, or mushroom.
- Gondi dramatically affects human beings, causing an increase in suicide, and other mental illnesses. One study showed an increase in children of infected with T. Gondi.
- Microbes can become more aggressive and cause illness in those with too much or too little sleep.
- Spinochordodes tellini influences the grasshopper’s brain making them go to water where the parasite invades and produce eggs.
- Schistolcephalus solidus infection makes stickleback fish move to colder water where the infection increases.
- Infections make animals seek higher ground to help predators find them
- A virus causes crickets to mate increasing transition of the virus.
Microbes Influence Stress and Anxiety

The first studies showing microbe’s effects on stress used a special variety of sterile mice that were experimentally stressed. Without microbes, there is a much higher level of stress steroids from the hypothalamus (corticosteroids) and a lower level of BDNF, (brain derived neurotrophic factor stimulates new neurons and brain connections). If these mice are given a particular set of microbes (bifdobacterium infantis), they become normal. If they are given E coli, they don’t become normal. Therefore, particular microbes have particular relationships with stress centers of the hypothalamus, pituitary, and adrenal, affecting behavior.
 Without stress, sterile mice have less anxiety in general, compared to mice with microbes but not pathological strains. These sterile mice spend more time in the light and are more sociable. Adding microbes without pathogens they become normal mice. But, not all species work. Some strains of microbes produce more anxiety than others. Different probiotics have very specific effects. Some probiotics or specific diets alter microbes affecting anxiety, depression and learning in mice. Lactobacillus rhamnosus lowered anxiety and depression, unless the vagus is cut. Two other species decrease anxiety and depression symptoms in mice as much as using an antidepressant.
Without stress, sterile mice have less anxiety in general, compared to mice with microbes but not pathological strains. These sterile mice spend more time in the light and are more sociable. Adding microbes without pathogens they become normal mice. But, not all species work. Some strains of microbes produce more anxiety than others. Different probiotics have very specific effects. Some probiotics or specific diets alter microbes affecting anxiety, depression and learning in mice. Lactobacillus rhamnosus lowered anxiety and depression, unless the vagus is cut. Two other species decrease anxiety and depression symptoms in mice as much as using an antidepressant.
Four weeks of probiotics (using four species of microbes) altered human reactions to emotional stimuli measured by MRI. With emotional stimulation, several key brain centers (primary interoceptive and somatosensory regions) did not have as much activity in response to pictures of emotional faces. The subjects stated they had less sadness and aggression. Another study of 50 people had less anxiety and less cortisol.
 A study showed that it was not a change in microbe species that made a difference, but rather a changed genetic production of protein and metabolism that correlated with the changes. It is possible that small numbers of specific microbes can make most of the difference.
A study showed that it was not a change in microbe species that made a difference, but rather a changed genetic production of protein and metabolism that correlated with the changes. It is possible that small numbers of specific microbes can make most of the difference.
Since microbes are vital for creating essential nutrients, do they, also, affect eating and drinking behavior? When we eat complex carbohydrates, the metabolic results of fermentation are able to go into the brain through the blood brain barrier. One of the products, acetate, affects the hypothalamus through multiple neurotransmitters to stimulate hormones to reduce eating. Thus, the microbe behavior of creating acetate through fermentation decreases appetite.
Microbe Effects on Social Behaviors
Mice without microbes are not as sociable as normal mice. They avoid others. Those mice with normal microbes are much more sociable. If mice without microbes who are socially abnormal, are given microbes as adults, they become normally sociable, but don’t remember comrades those that they were close to as children.
Therefore, microbes have different effects at different times in development of the brain. There is a critical window of time in children where colonization of microbes has to take place for normal development. Germ free mice had less BDNF and a decrease of important nerves in cortex and hippocampus, as well as cognitive loss and less working memory.
Importantly, when germ free mice were given the specific normal microbes of their strain they showed behavior related to the specific variety of microbes, not their specific species of mice. Other research shows that the individual type of microbe determines the susceptibility to anxiety and depression.
In flies with a particular microbe, they only want to mate with others who have that microbe. This occurs because of a smell from the microbe. In groups of hyenas, groups identify their social groups by a particular smell from microbes they carry. This influence can affect the ultimate evolution of the species.
Autism includes unique social deficits and, in addition, is associated with a higher amount of gut problems including different sets of microbes. Mice with autistic like symptoms have very different microbe sets in their gut and in addition have more of the microbe products in the blood. One particular microbe product, alone (4EPS), produced anxiety behavior. The mice in this experiment were given a particular microbe (Bacteriorides fragilis) and did better. These particular mice did better in behavior with less anxiety and social deficits. B. Fragilis helped normalize blood products that were associated with autism behaviors in animals. It normalized the 4EPS and anxiety.
Another study of mice with features of autism had gut inflammation from altered microbes. This might explain the sudden increase of autism in our society, which could not be a sudden genetic change. The altered lifestyle and microbes could be a factor.
Another change in mental status is the confusion (encephalopathy) that arises from severe liver disease. Some of this can be reversed with antibiotics to eliminate particular microbes that appear to be responsible for inducing more confusion. Psychiatric diseases also appear to be related to irritable bower disorder.
Microbe Gut Brain Connection
The gut is the “forgotten organ” of 100 trillion microbes—more cells than are in the rest of the body and 300 times as much DNA. Many molecules manufactured by the microbes are vital for humans, such as vitamins. But, with such a great amount of DNA to work with, most neurotransmitters are included and a vast number of manufactured factors are yet unknown.
Microbes have many critical functions in the gut nervous system. Recently, it was found that microbes regulate the critical migration of new glia cells in the enteric nervous system. New glia are produced in the ganglia of plexus in the gut nervous system. These cells then travel to the mucosa near the lumen of the GI tract. The recent research showed that microbes regulate this migration.
The enteric nervous system for the gut and its many related organs is the second largest group of neurons and glia and constitutes the second brain. This second brain has a much greater amount of neurotransmitters than are in the brain. It is semiautonomous, making many different hormones and signals.
The most important nerve between brain and gut is the vagus nerve, which is bi directional highly connected with gut motility, heart rate and breathing. Sensory is 80%. The vagus effect can be anti inflammatory, protecting against microbe blood infections through the nicotinic alpha7 nerves.
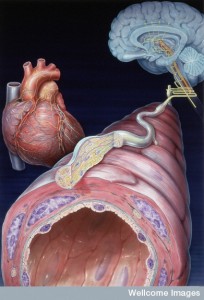 Communication from the gut to the higher centers has many important effects on cognition, motivation and emotions through the cortex, amygdala and many other brain centers. Microbes affect these centers either through direct secretion of signals or stimulation of the enteric nervous system and the large vagus nerve. The
Communication from the gut to the higher centers has many important effects on cognition, motivation and emotions through the cortex, amygdala and many other brain centers. Microbes affect these centers either through direct secretion of signals or stimulation of the enteric nervous system and the large vagus nerve. The
The two major branches of the autonomic nervous system, the sympathetic and parasympathetic, are connected with all major organs and brain centers. They are highly correlated with emotional states of stress, fear and calmness. The spinal reflexes and the nerves in the hypothalamic adrenal pathways are very important in both immune and emotional systems and communicate with the hypothalamus and amygdala.
There are many complex signals in the brain-gut-microbe axis related to food. Spinal cord nerves, the vagus nerve, and endocrine products including products from microbes all send nutrient signals. For the development of neurotransmitters, there are windows of time in development of the brain, which later becomes permanent. Many factors respond to differences in the microbes in an active way during childhood and adulthood.
Mice Without Microbes Have Altered Neurotransmitters
 Mice without microbes have decreases in several important neurotransmitters and factors. BDNF is lower, which affects the development of new brain cells for memory. Serotonin is less in critical brain regions, hippocampus and amygdala. If microbes are added later, these neurotransmitter levels do not increase to normal.
Mice without microbes have decreases in several important neurotransmitters and factors. BDNF is lower, which affects the development of new brain cells for memory. Serotonin is less in critical brain regions, hippocampus and amygdala. If microbes are added later, these neurotransmitter levels do not increase to normal.
These mice without microbes have alterations in the striatum with rapid production and turnover of serotonin, dopamine and norepinephrine. This increases blood flow to muscles and neurons increasing movement. They, also, have increased blood brain barrier leakage during their entire life. A particular bacteria clostridium tyrobutyricum alone will restore the brain barrier function even in adults. It is the metabolite butyrate grom the gut that alone restores the barrier function. This shows that ongoing signals from the products of the microbes affect the blood brain barrier. It is unusual to consider that regulation of the blood brain barrier occurs in the gut, which is no where near the cerebrospinal fluid.
It is not known what these changes in the blood brain barrier allow into the brain and what other signals this can create, since microbes produce a wide variety of other metabolites that can serve as signals. Metabolites of microbe fermentation produce products that send a signal to the cells lining the blood brain barrier. These signals make the barrier tight and don’t allow any random molecules to enter the brain. When these products are not present, the the barrier opens and allows other molecules into the brain. (see post on T cells)
Microbes Directly Affect Neurotransmitters
 Of the 3.3 million genes in the bacteria, many are able to create psychoactive signals and some generate the same neurotransmitters and modulators of neurons. Individual species of microbes produce GABA, noradrenaline, serotonin, dopamine and acetylcholine the major neurotransmitters that effect emotion. Microbes produce tryptophan and kynurine when stimulated by inflammatory factors and steroids.
Of the 3.3 million genes in the bacteria, many are able to create psychoactive signals and some generate the same neurotransmitters and modulators of neurons. Individual species of microbes produce GABA, noradrenaline, serotonin, dopamine and acetylcholine the major neurotransmitters that effect emotion. Microbes produce tryptophan and kynurine when stimulated by inflammatory factors and steroids.
Microbes influences affecting anxiety and depression are demonstrated by probiotics affecting specific neurotransmitter production. Other microbes influence with cell wall sugars. These sugars are a significant mechanism of probiotic microbes with an outer coating protecting human cells from acids and bile, and shielding microbes from immune response. Cell wall components stimulate epithelial lining cells to release signal molecules that act on primary afferent axons.
When there are no microbes in the gut, there is less serotonin in the blood and less serotonin metabolites and molecules that are made into serotonin in the gut. Surprisingly, only a small amount of serotonin is produced in the raphe nucleus of the brain. Rather, 90% is produced by cells in the gut nervous system to regulate intestinal movements. The same fermented products of complex carbohydrates stimulate serotonin from these gut cells. (Enterochromaffin cells). Since serotonin doesn’t cross into the brain from the blood, the important precursors can affect the levels of brain serotonin. Tryptophan is produced by microbes of the gut, absorbed into the blood and then crosses the blood brain barrier. There it is metabolized into serotonin.
The increase of serotonin in the gut affects the muscle movements of the gut. A very particular set of microbes has been found that brings serotonin back to normal as well as regulating platelets and clots (serotonin is involved in modulating platelets). These specific microbes stimulate the gut cells to make more serotonin. It remains to be seen whether probiotics can perform the same functions as antidepressant medications that increase serotonin at the synapses.
Without microbes in the gut, the levels of dopamine and GABA are, also, decreased. Another complex set of circumstances affects molecules that are metabolized into these neurotransmitters and those that are produced by metabolizing them. These processes are not entirely clear but might involve the same cells that manufacture serotonin, or neurons and glia in the gut nervous system.
 Also, microbes make many other small molecules that can be neuro modulators or new neurotransmitters. This includes serotonin, dopamine, GABA, epinephrine, acetylcholine and others. These can go into the blood and the CSF. But, their specific function is not clear. One specific microbe makes tryptamine, which inhibits serotonin effects and affects mood and eating. Tyramine is another neurotransmitter made by several specific microbes, which affects muscle function.
Also, microbes make many other small molecules that can be neuro modulators or new neurotransmitters. This includes serotonin, dopamine, GABA, epinephrine, acetylcholine and others. These can go into the blood and the CSF. But, their specific function is not clear. One specific microbe makes tryptamine, which inhibits serotonin effects and affects mood and eating. Tyramine is another neurotransmitter made by several specific microbes, which affects muscle function.
GABA is manufactured by microbes and triggers the vagus nerve and travels in the blood. GABA is a critical factor for many aspects of intestinal function including its movements, emptying and the secretion of products into the lumen of the gut. It is, also, very involved in the response to stress and changes in temperature.
It is not widely known that there are a greater amount of many neurotransmitters in the gut, than in the brain. This large amount of active signaling molecules that enters the blood can have effects on the vagus nerve and other parts of the nervous system that are not yet known. The large complex vagus is the major nerve between the central nervous system the gut nervous system.
Microbe Effects Through the Immune System
 Other posts have described the massive connections between the immune system and the nervous system. This cross talk is critical in pain, depression, degenerative brain disorders. A previous post described how T cells in the cerebro spinal fluid send wireless signals to neurons to maintain cognition, until there are microbes noticed. Then T cells trigger the “sick feeling”, a mental state that allows for more rest and healing. Sick feelings include decreased appetite, decreased movement and use of energy and decreased social behavior and cognition.
Other posts have described the massive connections between the immune system and the nervous system. This cross talk is critical in pain, depression, degenerative brain disorders. A previous post described how T cells in the cerebro spinal fluid send wireless signals to neurons to maintain cognition, until there are microbes noticed. Then T cells trigger the “sick feeling”, a mental state that allows for more rest and healing. Sick feelings include decreased appetite, decreased movement and use of energy and decreased social behavior and cognition.
Increasing research in this area shows that a massive communication occurs once inflammation is triggered. Many different immune cells are activated and a wide range of powerful cytokines are triggered, which proceed to the brain. Many cytokines are transported across the blood brain barrier by special molecules, while some can cross without transporters. Cytokines trigger microglia, astrocytes and neurons (see post No Brain Mapping Without Glia). These cytokines, also, greatly affect the nerves in the periphery and nerves that return to the CNS.
Recently, it was found that specific cytokines can mediate the sick behavior, while others don’t. Cytokines can affect many different neurological symptoms. In treatment with IL-2 and INF? for cancer, there is a very high amount of depression.
In the mice models of autism there are many immune abnormalities, with less special T cells and increased cells of inflammation. Radiation treatment for cancer affects the microbes with some improvements in behavior symptoms. Transplantation of new immune cells helps improve the autism symptoms.
Inflammation can affect behavior in other ways, such as triggering cytokines. Several cytokines alter the molecule that transports serotonin back into the cell eliminating it in the synapse. This lowers the amount available to trigger the synapse. These same cytokines, also, decrease the production of receptors for the serotonin on the post synaptic neuron. Both factors cause cause depression and the sick feeling.
 Some microbes directly produce signals that increase the release of cytokines that increase inflammation. Without microbes decreased factors don’t go through the blood brain barrier to stimulate new neurons. This is part of the cause of decreased new neurons in depression. This same pathway can affect degenerative brain diseases. In the future specific microbes that stimulate this pathway could increase new neurons.
Some microbes directly produce signals that increase the release of cytokines that increase inflammation. Without microbes decreased factors don’t go through the blood brain barrier to stimulate new neurons. This is part of the cause of decreased new neurons in depression. This same pathway can affect degenerative brain diseases. In the future specific microbes that stimulate this pathway could increase new neurons.
It was once thought that the womb had no microbes. Now, it is thought possible that microbes might influence fetal brain development. Also, microbes in the mother can affect blood levels of factors and cytokines, which can affect fetal brain development.
Another important question is the effect of changes in microbes with disease and how this affects the brain. Evidence of environmental factors affecting degenerative brain disease might be related to microbe pathways.
Probiotics
Probiotics are a form of medicine where positive microbes are introduced to fight disease. But, many different microbes have very different probiotic effects. These are just now beginning to be catalogued and will undoubtedly lead to many future medical treatments.
Probiotic bacteria compete for dietary ingredients, convert sugars into fermentation products with inhibitory properties, produce vitamins and other growth substances, produce substances toxic to other bacteria, and compete for neurotransmitter binding sites.
Probiotics can influence opiod and cannabinoid receptors, which may affect pain. Probiotics have helped with irritable bowl syndrome, anxiety, stress and mood. These appear to work by interfering with the cytokines that create sickness behavior and depression, and anxiety. The vagus nerve is critical for some probiotic effects.
In a mouse model of depression where children separated from mothers early in life, a probiotic eliminated the depressed behavior and altered noradrenaline and CRF in the brain. In mice with colitis the same probiotic was able to eliminate anxiety and normalize hippocampus BDNF.
Microbes Effect on the Brain
Microbes in the gut have 300 times more DNA than in human cells. Products from this extra microbial DNA produce a vast array of possible effects on the brain. The many mechanisms are just being discovered.
Microbes can produce precursors of neurotransmitters and hormones. They can, also, directly produce these factors. They can affect the immune system’s cytokines to influence the brain. They can affect the blood brain barrier and allow factors into it. And they can directly travel to the brain and invade it.
Friendly microbes live for decades in peaceful cooperation with human cells and help to fight dangerous bacteria and viruses. They provide completely vital functions needed for human life. They are critically involved in the development of the brain and brain functions in adults.
With such a vast influence of these multiple communities of cells, it has to be asked how this can all be coordinated and directed to form a human being.