 Recent research into the causes of Alzheimer’s has become increasingly complex. For a long time it was assumed that buildup of amyloid-β causes destruction of neurons and, therefore, the degenerative brain disease. Most current drug trials are trying to eliminate the toxic form of amyloid. However, very recent research has shown that many (possibly 30%) of Alzheimer patients have no such buildup and many normal elders do have amyloid in the brain (See post on Amyloid and Alzheimer’s). Therefore, amyloid as a cause of Alzheimer’s is not at all certain.
Recent research into the causes of Alzheimer’s has become increasingly complex. For a long time it was assumed that buildup of amyloid-β causes destruction of neurons and, therefore, the degenerative brain disease. Most current drug trials are trying to eliminate the toxic form of amyloid. However, very recent research has shown that many (possibly 30%) of Alzheimer patients have no such buildup and many normal elders do have amyloid in the brain (See post on Amyloid and Alzheimer’s). Therefore, amyloid as a cause of Alzheimer’s is not at all certain.
Tau is a critical protein that holds together the very active microtubules that build and rebuild structures in the axon and dendrite (see post on axon transport). A very complex relationship of tau, amyloid-β and inflammation is emerging as possible causes of Alzheimer’s. Tau, itself, appears to be correlated with many other forms of brain disease. However, the latest research with tau is not at all clear or simple, either. The clumps don’t exactly correlate with Alzheimer’s, but the transmission of tau (of some unknown type) along specific neuronal circuits does. In fact, these circuits map out the exact development of Alzheimer’s in the brain.
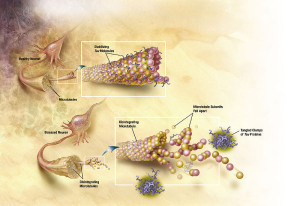 It is not known whether the travelling destructive toxic tau consists of aggregates (probably not), small oligomers (several stuck together), or other tau molecules that are soluble in water. It is known that there are many different kinds of abnormal tau and many different kinds of brain diseases are related to these. There are now known that there are a large number of different tags on tau, similar to epigenetic tags on DNA and histones, all of which have very complex properties. One of the current barriers to research is that the small oligomers of tau cannot yet be tagged and compared to soluble tau.
It is not known whether the travelling destructive toxic tau consists of aggregates (probably not), small oligomers (several stuck together), or other tau molecules that are soluble in water. It is known that there are many different kinds of abnormal tau and many different kinds of brain diseases are related to these. There are now known that there are a large number of different tags on tau, similar to epigenetic tags on DNA and histones, all of which have very complex properties. One of the current barriers to research is that the small oligomers of tau cannot yet be tagged and compared to soluble tau.
This post will summarize the latest, but very incomplete and confusing, information on normal and toxic tau in the brain. It will describe the role of tau in brain function and dementia.
Tau Protein in the Neuron
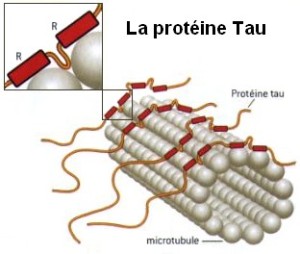
Tau is an unusual protein in that it is often unfolded, unlike most proteins that have highly specific shapes determining their functions. (There is new emerging research finding a variety of proteins have flexible shapes.) Tau is critical for stabilizing microtubules in the neuron, which have been described in previous posts as the LEGO brain of the neuron, constantly building and rebuilding axons and dendrites in response to thought. Normal tau is soluble in water and doesn’t naturally combine with other tau molecules to form aggregates. An abnormal type of tau is part of the destruction of the brain in Alzheimer’s.
Tau is called a “microtubule associated protein”, one of many stabilizing and regulating the vast amount of shapes that microtubules form.
The gene that makes tau is called MAPT for microtubule-associated protein tau gene. It has 16 exons (the separate pieces of DNA that are edited together) on a particular chromosome (17q21). This gene makes tau in neurons and a small amount in glia. By alternate splicing of exon 2 (E2), E3 and E10, that is, editing the 16 pieces of DNA in different ways, 6 different types of tau are present. A previous post noted that the human brain uniquely uses alternative splicing among all other animals. Thus far, only the alternate editing of exon 10 is known to be associated with abnormal tau and brain diseases. These diseases can be divided into three distinct patterns of repeated sections called R3 and R4. Alzheimer has both 3R and 4R and each of the others have either one or the other.
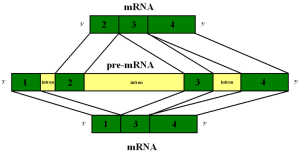 The alternative splicing of the exons can occur because of mutations on the MAPT gene, but, also, other factors, such as proteins that bind various RNAs. In the fetus, only one of these is produced. Different regions of the brain have different amounts of the six normal types of tau and different kinds of splicing. The cortex has twice the amount of tau messenger RNA than the cerebellum and white matter. There has been research that shows certain factors can influence which are produced. One product is related to mitochondria.
The alternative splicing of the exons can occur because of mutations on the MAPT gene, but, also, other factors, such as proteins that bind various RNAs. In the fetus, only one of these is produced. Different regions of the brain have different amounts of the six normal types of tau and different kinds of splicing. The cortex has twice the amount of tau messenger RNA than the cerebellum and white matter. There has been research that shows certain factors can influence which are produced. One product is related to mitochondria.
Tau binds to RNA by electro chemical interactions and it may help stabilize RNA and DNA structures in the nucleus, as well as microtubules. It is possible that this interaction with RNA is part of the problem that forms aggregates of abnormal tau. One of the tau variants (4R) is better at helping microtubules.
One particular region of the tau protein that faces away from the attachment to microtubules is called the projection domain. This section of tau is influential in determining the space between two microtubules that are stabilized by tau. Different versions of this N-terminal of the amino acid affects where the different tau are located inside the cell and, also, are related to which will form tangles.
One problem of animal research with tau is that mice only produce half of the 6 types of tau. Also, they are different in the N-terminal header section.
Normal Tau Functions
 Until recently, the function of tau was thought to be only stabilizing microtubules. However, its particular role in this regard seems to be regulating the instability of the microtubule structures. This allows the cytoskeleton to rapidly change structures in the axon and dendrite. There are only small areas of the tau molecule that actually bind to the α and β-tubulins of the microtubule. So, the rest of the molecule remains flexible and changeable for new shapes. In fact, the normal regions, which become part of abnormal clumps, usually form a hairpin structure that looks like a paper clip.
Until recently, the function of tau was thought to be only stabilizing microtubules. However, its particular role in this regard seems to be regulating the instability of the microtubule structures. This allows the cytoskeleton to rapidly change structures in the axon and dendrite. There are only small areas of the tau molecule that actually bind to the α and β-tubulins of the microtubule. So, the rest of the molecule remains flexible and changeable for new shapes. In fact, the normal regions, which become part of abnormal clumps, usually form a hairpin structure that looks like a paper clip.
Tau, also, regulates transport along the microtubules, influencing dynein and kinesin motors’ movement in both directions toward the cell body and toward the terminal end of the axon at the synapse. It competes with these motors regulating their speeds and movements. It slows them down for pickups, such as mitochondria. Tau is, also, a cargo itself and competes with other types of transport. Tau, also, regulates release of vesicle cargoes. It interacts especially with dyactin a cofactor of dynein motor. But, the real influences are not fully understood. Tau is needed for axons to grow. (See post on transport along the axon).
Recent research demonstrates a definite effect on neuroplasticity and long-term potentiation, but the mechanisms are not yet clear. It, also, interacts with the proteasome, a structure that grinds up used proteins to recycle the material.
In Dendrites and Cellular Nucleus
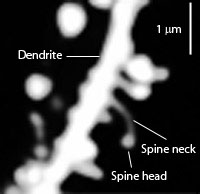 Tau might be involved in the different shapes of dendritic spines, but this not entirely clear. It is definitely involved in neuroplastiticy in the dendrite. Tau, also, exists in the nucleus of neurons and fibroblasts and in neuronal stem cells.
Tau might be involved in the different shapes of dendritic spines, but this not entirely clear. It is definitely involved in neuroplastiticy in the dendrite. Tau, also, exists in the nucleus of neurons and fibroblasts and in neuronal stem cells.
Tau appears to be important in the complex structure of DNA in the nucleus (see post), the positions of RNA in the nucleus and the cytoplasm. More is being learned about how the exact locations of chromatin in the nucleus are, in fact, critical to which parts of DNA are utilized. Tau is involved in stabilizing and regulating this internal three dimensional nuclear structure.
Tau is involved in regulating the activity of neurons during migration. It is involved in the transport of iron in the neuron. Tau, also, interacts with the actin structures, which form many of the other structures in the changing neuron.
Structure of Tau
Tau is unusual among proteins for being so water soluble, which is based on the fact that most of the amino acids are friendly to mixing with water. Tau is, also, unusual in that it doesn’t mind acids and high temperatures, because it has some acidic amino acids. In fact, it has various different regions that have different properties of charge influencing its actions with microtubules, its folding and the formation of bundles.
Tau lives mostly inside cells, but is secreted from neurons under some conditions. The more electrical and synapse activity in the neuron, the more tau is released outside the cell. There is no evidence that it is released in synaptic vesicles or is related to the ER and Golgi. But, it is possible that other types of vesicles, for example exosomes, or secretion pathways are involved. Different types of tau occur in cerebral spinal fluid and interstitial fluid (in mice) without brain disease.

Extracellular tau, including the abnormal types, are cleared from the brain by the glymphatic system (see post Five Secrets of Brain Health) and by a special complex called the proteasome that grinds up mis folded proteins for material that is reused. Abnormal tau is involved in blocking this proteasome process, inhibiting clearance of abnormal proteins in the brain.
After secretion from the neurons, tau can stimulate some neurotransmitters including acetylcholine M1 and M3 increasing calcium, which can kill cells.
There are two sections of tau —the C-terminal assembly domain and the N-terminal projection domain. The C or carboxy end binds to microtubules. A region that links the two ends together is related to a tyrosine kinase called FYN, which may be related to Alzheimer’s.
Tau is unfolded naturally, but might take specific shapes when binding to microtubules or from other factors associated with building microtubule structures. It likes to take a shape like a paper clip, where all the sections are near each other. When abnormal tangles are formed, it is the middle section that combines with others, not the terminals, which don’t combine and stay flexible. Abnormal tau doesn’t have the paperclip form, which alters its function.
Adding of Phosphate to Tau
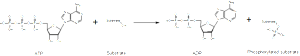
In the fetus, tau has seven phosphates attached (phosphorylation), whereas adults only have two. In Alzheimer’s, the number of attached phosphorus particles increases to eight, at least. There might, in fact be more phosphates, but most studies are from cadavers, which could have lost some of them through enzyme actions. It does appear that the number of attached phosphates at particular regions is what makes tau abnormal.
In fact, there are 85 places where phosphates can attach and 45 have been seen in research. Two sites seem to be related to the brain diseases (17 Thr-Pro or Ser-Pro) and are related to enzymes that work with serine, proline and threonine amino acids. The type of enzyme that places high-energy phosphorus particles on molecules is called a kinase. Kinases are one of the largest families of proteins in all of nature. There are many different kinases involved in this process and there are variations in what section is phosphorylated in Alzheimer’s.
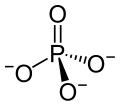 An opposite type of enzyme takes phosphorus tags off of molecules and there are many of these phosphatases, also. One in particular (PP2A) does 70% of this activity in the human brain and there is less with Alzheimer’s (20% less in the grey matter or cell bodies and 40% less in the white matter or axons.) There are a lot of different ways this could happen including changing the enzyme, more inhibitors or other genetic factors. Phosphatases are all more sensitive to low temperature, which inhibit it much more than kinases. Going along with this line of thinking, there are less phosphate groups when animals hibernate and during anesthesia hypothermia.
An opposite type of enzyme takes phosphorus tags off of molecules and there are many of these phosphatases, also. One in particular (PP2A) does 70% of this activity in the human brain and there is less with Alzheimer’s (20% less in the grey matter or cell bodies and 40% less in the white matter or axons.) There are a lot of different ways this could happen including changing the enzyme, more inhibitors or other genetic factors. Phosphatases are all more sensitive to low temperature, which inhibit it much more than kinases. Going along with this line of thinking, there are less phosphate groups when animals hibernate and during anesthesia hypothermia.
Phosphorylation is a critical part of normal regulation of tau activity and influences microtubule assembly and stabilization. Adding more phosphate to one region in tau changes its attachment to microtubules. Another regional change causes release of tau from the microtubule. Another changes the shape of the tau altering its properties—this is called a trans to cis shift. A different enzyme brings it back to trans re attaching the tau to the microtubule. Cis tau is found in Alzheimer’s brains and after traumatic brain injuries.
There are other ways that more phosphates can cause problems. More phosphates shift some tau from axons to the neuron cell body and the dendrite altering the synapse. More phosphates can, also, change how tau is metabolized as well as its shape. Phosphates in one spot stops the normal cutting of the molecule for recycling (by caspase enzyme). It is possible that it increases formation of clumps and fibrillary tangles. It can alter how it functions with some of the many other factors holding the microtubules together, such as kinesin related proteins. This will stop the formation of the complex that allows the kinesin motor to function on the microtubules. This alteration changes the transport along the axon (see post).
Acetylation and Other Tags on Tau
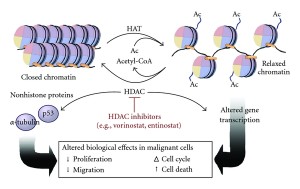
The addition of phosphates on tau has been understood for some time. It has been assumed to be critical, but the details are just emerging. Another tag—acetyl—has just been discovered and might, also, be relevant. Acetyl is one of many tags in epigenetic tagging of DNA and histones, as well. One of the same enzymes that operates with histones, also, works with tau. There are now several different enzymes found to place acetyls on tau and another set of enzymes that take them off (acetyltransferase and de acetylases).
What is unusual is that the molecule tau itself can act as an acetyltransferase and place acetyls on itself. This could affect the normal ability to clear out and metabolize tau, both decreasing and increasing it.
There are at least 4 places where acetyls are placed on tau and these are decreased in Alzheimer’s. Another site is greater with Alzheimer’s and other tauopathies. A second place stops metabolism of tau and appears to be correlated with toxic tau

As epigenetic tagging of DNA and histones has become extremely complex with more than 40 types of tags presently known, there are now many more tau tags as well, called post translational modification. These include glycosylation, glycation, deamidation, isomerization, nitration, methylation, ubiquitination, sumoylation (see post) and truncation.
In Alzheimer’s, there is unique N-glycosylation, which appears to increase phosphates on tau as it changes its shape. O-glycosylation does the opposite and stops fibrillary tangles. This latter type occurs less in Alzheimer’s, which may be an important factor in increasing tangles and destruction of neurons. The mechanism doesn’t seem to be just blocking sites on the tau molecule, but some more fundamental process. There are, in fact, multiple other tags that are present in toxic tau, and not the normal variety. So, it is possible that there are many different factors that create fibrillary tangles. Some of these alter the way tau binds to microtubules.
The addition of nitrates to tau in several spots exists in normal people and not with dementia. But several other locations, the opposite occurs. These can affect binding to microtubules, either increasing or decreasing the binding based on where the tag occurs.
Methylation in several places stops tangles. Alzheimer’s patients have less methylation.
Ubiquitylated and sumoylated tags occur, but their significance is not clear. A previous post described these very important complex tags that are used for many molecules and many different purposes. In fact, cells and microbes engage in fierce batter altering these tags back and forth
Another process, called truncation, shortens the tau by stopping the messenger RNA from making the full tau molecule. This shape alteration stops the paperclip structure and helps form clumps. These fragments can cause neuronal damage without clumping. Some truncations make the attachment to microtubules stronger along with many tags.
Sorting in Compartments
During fetal life, tau exists in all areas of the neuron, but later mostly in axons and a little in dendrites. In dendrites, tau is metabolized faster. Tau is made in the cell body and rapidly transported by the microtubules to the axons. There is a barrier to send them back into the dendrites. Also, different forms of tau appear in different places. In Alzheimer’s, tau shows up in the dendrites. There are probably different functions in different regions. Recently, it has been found in the nucleus.
Abnormal Tau in Brain Disease
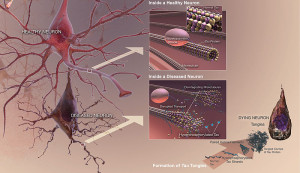 As the various forms and mutations of tau become known, the toxic element is more elusive than before. It is not clear that tau aggregates, either a small number as oligomers, or larger as fibrils, are, indeed, the cause of brain disease. It is possible that a soluble variation, not a clump, is creating the toxicity. The complexity is growing greater with each study.
As the various forms and mutations of tau become known, the toxic element is more elusive than before. It is not clear that tau aggregates, either a small number as oligomers, or larger as fibrils, are, indeed, the cause of brain disease. It is possible that a soluble variation, not a clump, is creating the toxicity. The complexity is growing greater with each study.
The abnormal tau forms small “paired helical filaments” PHFs and large “neurofibrillary tangles” NFTs, which are present in many different brain diseases called tauopathies, including Alzheimer’s, Pick disease (PiD), progressive supra nuclear palsy (PSP), Huntington’s disease (HD), frontotemporal dementia with parkinsonism-17 (FTDP-17), corticobasal degeneration(CBD), and agryrophilic grain disease (AGD). However, it is not clear how tau is acts in these diseases and they don’t always correlate with disease.
In fact, there are many types of PHFs, including twisted and untwisted types and those in pairs and combinations of more. Also, some form of abnormal tau is now known to be transmitted between neurons in specific circuits.
Causes of Abnormal Tau
A tremendous amount of research is attempting to find how abnormal tau causes brain diseases, but this is still unclear. There are many possibilities. In disease, many of the tags occur that break the attachment of tau with microtubules. With this disruption, pieces of tau enter the synapse and disrupt the vesicles. It can also accumulate in the dendrite and further into the body of the neuron. When disrupted, tau doesn’t enter the nucleus to help with the structure of DNA, which becomes disrupted.
Tau clumps can be released from the neuron in vesicles to be taken up by other neurons in a circuit of destruction. This uptake can take many forms, such as exosomes or endocytosis.
Another recent development is the discovery of a proteasome complex in the cell that grinds up and cleans out mis folded proteins, which are then used as amino acid material for other proteins. Tau is part of the structure and appears to stick to the proteasome, stopping it from functioning. In this process, tau contributes to accumulation of mis folded proteins. A drug called Rolipram appears to restore functions of the proteasome by altering and restoring its shape after interference by toxic tau.
Tau Mutations
Eighty different tau mutations have been discovered and occur in many of the tauopathies. Some change the sequence of peptides and some the alternative splicing. Most of the first types are in the region that binds to the microtubule and these often leads to clumps. Another makes the structures less efficient and makes small clumps only, not large fibers. The splicing type makes more 4R and leads to aggregates.
Tau Aggregates
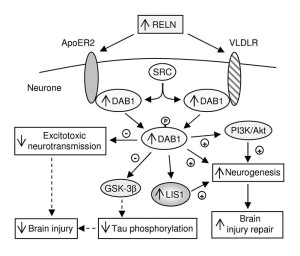
While fibrils occur in many brain diseases, smaller clumps, also, occur in the disease PART or primary age related tauopathy. It is not clear if this is an early stage of dementia or not. Most of the tau molecule is free flowing with only several regions that form β-sheets that can form clumps. β-sheets are present in many proteins and are one way that proteins form larger structures. When β-sheets can be stacked together it leads to clumping through inter digitation of the sheets. Some chemical factors increase clumping in research, but it is not clear how these relate to pathology.
The one factor that seems to cause clumps is the attachment of too many phosphate molecules, or hyper phosphorylation. But, there are so many different sites for phosphate attachments, it is not yet clear if this is a cause of aggregation or effect. There are many other kinds of tags that are described above. Some phosphorus sites protect against the clumping. Some regions form only small clumps not fibrils. In anesthesia and animal hibernation, the same increased phosphorylation occurs as in Alzheimer’s, but there are no clumps formed. So, this is not a total cause. Truncated tau has more tendency to form clumps by loss of the paperclip form.
One theory is that a special nucleus has to form in the small clumps and then this will proceed to larger clumps. This is called nucleation-elongation mechanism. In research, injection of some forms of tau starts the spread to other neurons like prions. It is not yet clear if tau acts just like a prion in that a mis folded form of tau causes normal tau to change into the abnormal shape forming clumps and being transmitted. More phosphorylation breaks up microtubules. It is not known whether the new functions, such as with DNA are relevant.
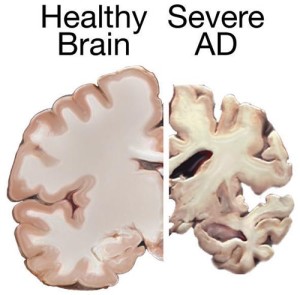 The amount of neurofibrillary tangles (NFTs) is somewhat correlated with Alzheimer’s pathology, but not yet proven to be a cause and it is not in everyone. (See the post on Amyloid for more on this.)
The amount of neurofibrillary tangles (NFTs) is somewhat correlated with Alzheimer’s pathology, but not yet proven to be a cause and it is not in everyone. (See the post on Amyloid for more on this.)
Many Alzheimer’s brains have neuron destruction, without NFTs. Neurons can survive 20 years with NFTs, and most neurons die without NFTs. Therefore, it is possible that soluble abnormal tau is the problem not just the NFTs. In fact, the NFTs might be the cell’s way to protect against soluble toxic tau. The NFTs can alter the cell’s structure isolating components that need to communicate, such as interfering the axon transport.
Soluble Toxic Tau
Some research in Alzheimer’s is directed at soluble amyloid particles not plaques as most important to damage the neurons. In the same way, recent research points to small particles of abnormal soluble tau being the toxic particle. But, this research is controversial.
Seeding and Spreading Toxic Tau
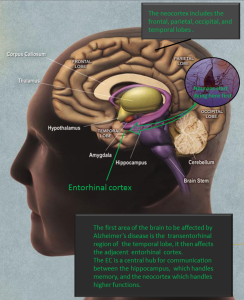
Tau neuronal damage in Alzheimer’s starts in the entorhinal cortex and spreads to the hippocampus, then other regions of the cortex. It is not clear what causes this spread, but it may be related to inflammation.
The fact is that the pattern of Alzheimer’s is the same as the spread of tau in circuits. But, it is not known which type of tau is spreading and what starts this process. The seeds can be from vesicle transmission with exosomes (See post on Exosomes), which travel and merge with other neurons. Another process could take in extracellular tau seeds by the endocytosis process, similar to taking in microbes and nutrient materials. It could, also, seep across synapses.
Perhaps, the biggest problem with current research is the inability to label small bunches (oligomers) of tau and compare it with single molecules of soluble tau. Tau could, also, be toxic for other reasons. The localization seems to be important and tau in the dendrite is related to amyloid toxicity.
Future Possible Treatments Related to Tau
 Given the various possible mechanisms described, there are many avenues of possible treatments. One is the reduction of tau, but, in fact, this increases amyloid-β and is not directly correlated to disease, but indirectly. Therefore the expression of the manufacture of tau might be relevant through treatment with anti sense nucleotides, microRNAs and interfering RNAs, and transcription factors.
Given the various possible mechanisms described, there are many avenues of possible treatments. One is the reduction of tau, but, in fact, this increases amyloid-β and is not directly correlated to disease, but indirectly. Therefore the expression of the manufacture of tau might be relevant through treatment with anti sense nucleotides, microRNAs and interfering RNAs, and transcription factors.
A second treatment could be inhibiting the addition of phosphorus onto tau molecules. But, as shown already, this is extremely complex and it is not known exactly which sites are relevant. Possibly inhibiting the correct kinase enzymes or stimulating the correct phosphatases could help.
There are other approaches:
- Another approach is to stop clumping with several possible agents.
- Stabilizing microtubules might help with several new agents.
- Affecting the cellular processes that clean out misfolded proteins (proteasome) or destroy infected cells (autophagy) might help. This might involve inhibiting chaperone molecules. Rapamycin stimulates autophagy and might help destroy toxic cells before they spread.
- Another avenue of treatment could be immunotherapy with vaccines and tau antibodies.
The Role of Tau in Brain Function and Dementia
As with everything else in the brain, the story of tau in healthy brains and disease is extraordinarily complex. There is clearly some relationship with amyloid–β, but it is not clear. It is related to inflammation, but this is not clear, either. Too many phosphorus tags appear to be relevant but there are too many different sites and effects to determine what this means. Now, with many other tags, the picture is extremely complex and confusing. Clumping appears to be important, but the correlation is not exact. In fact, it could still be found that the soluble version of toxic tau is the one that is transmitted along the Alzheimer’s circuit.
The one thing, for now, that seems certain is that the path Alzheimer destruction takes correlates with the transmission of some form of toxic tau. The rest is vastly complex as is everything else in the brain.