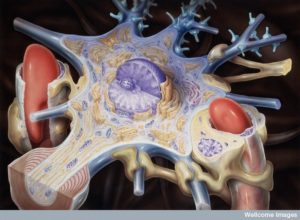
 Both the brain and the immune system perceive infections, trauma, stress, anxiety and social isolation. Both respond to efforts to defend and heal these traumas. Many previous posts have described increasing understanding of how neither can operate without the other. They have been called the “wired and wireless brains,”—wired for rapid transfer of information and response and wireless for hard to reach places and local responses. Many questions remain. This post is an update on the latest research in how T cells operate in the brain; how immune cells get into and out of the brain; positive and negative outcomes of immune cells in the brain; and the question of how the two systems evolved together.
Both the brain and the immune system perceive infections, trauma, stress, anxiety and social isolation. Both respond to efforts to defend and heal these traumas. Many previous posts have described increasing understanding of how neither can operate without the other. They have been called the “wired and wireless brains,”—wired for rapid transfer of information and response and wireless for hard to reach places and local responses. Many questions remain. This post is an update on the latest research in how T cells operate in the brain; how immune cells get into and out of the brain; positive and negative outcomes of immune cells in the brain; and the question of how the two systems evolved together.
 The previous generation of neuroscientists considered the brain “immune privileged” meaning that there were no immune cells in the brain. At that time, there was no way to understand how cells related to inflammation could be in the brain without infection. Very recently, with better imaging of individual cells in the vast brain, it has been found that there are normally many immune cells in the brain
The previous generation of neuroscientists considered the brain “immune privileged” meaning that there were no immune cells in the brain. At that time, there was no way to understand how cells related to inflammation could be in the brain without infection. Very recently, with better imaging of individual cells in the vast brain, it has been found that there are normally many immune cells in the brain
A previous post on T cells described how the signaling from T cells to brain cells is vital for both normal cognition and the altered “sick feeling” during infections. During illness, the brain is sent special signals to slow down and rest so that healing can occur. T cells send tonic pulses of signals to the brain to maintain ordinary cognition and to increase memory at times. T cells, also, respond to acute stress with increased signaling for memory and learning in a crisis situation. During chronic stress T cells send the opposite messages, decreasing cognition.
T cells in the cerebrospinal fluid and brain tissues have interactions with brain cells because they have an active antigen relationship, but of a special kind. There is a distinct antigen response to the brain tissues. Recent research detailing immune responses in the brain have described specific signals related to stress, anorexia, fever, and autoimmune diseases such as multiple sclerosis.
In the 1990s it was first noted that immune system cells do not just respond to infections, but to signals of danger from tissues with trauma. Later it was found that immune cells have vital roles in healing of all kinds and even maintenance of tissues in a normal state.
Interactions in Brain Disorder
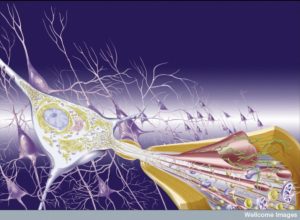 Multiple sclerosis and immune encephalitis are diseases where immune cells attack myelin both in the spinal cord and brain. Early research connected CD4+T cells as attacking myelin. They were reactive to proteins in the myelin—myelin basic proteins, proteolipid protein and myelin oligodendrocyte glycoprotein. Now it has been found that there are many other immune cells involved in this process. In multiple sclerosis, CD8+T cells, B-lymphocytes, neutrophils, natural killer cells, monocytes and macrophages all appear to be involved in very complex communication. Many of these cells regulate an inflammation that is particularly damaging.
Multiple sclerosis and immune encephalitis are diseases where immune cells attack myelin both in the spinal cord and brain. Early research connected CD4+T cells as attacking myelin. They were reactive to proteins in the myelin—myelin basic proteins, proteolipid protein and myelin oligodendrocyte glycoprotein. Now it has been found that there are many other immune cells involved in this process. In multiple sclerosis, CD8+T cells, B-lymphocytes, neutrophils, natural killer cells, monocytes and macrophages all appear to be involved in very complex communication. Many of these cells regulate an inflammation that is particularly damaging.
Increasingly, other neurodegenerative diseases are found to have immune processes as well. Previous posts described complex interactions involving microglia in multiple dementias (see post). While research is in its early phases microglia have been implicated in positive and negative effects related to brain inflammation and degenerative diseases.
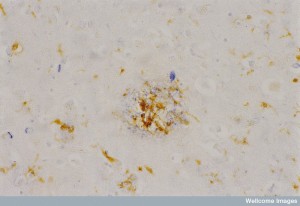 Alzheimer’s demonstrates unusual affects from microglia. But, also recently other types of immune cells called phagocytes (immune cells that eat debris) have been found to be involved in inflammation in Alzheimer’s. The specific types of phagocytes are not yet clear. Microglia are in the family of macrophages that have stayed in the brain since birth. These recently discovered phagocytes might be related to microglia or macrophages from monocytes that are brought into the brain from the blood. All of these are in the same myeloid line of cells.
Alzheimer’s demonstrates unusual affects from microglia. But, also recently other types of immune cells called phagocytes (immune cells that eat debris) have been found to be involved in inflammation in Alzheimer’s. The specific types of phagocytes are not yet clear. Microglia are in the family of macrophages that have stayed in the brain since birth. These recently discovered phagocytes might be related to microglia or macrophages from monocytes that are brought into the brain from the blood. All of these are in the same myeloid line of cells.
More recent research shows that cells that are not in the myeloid line may also be part of the Alzheimer picture. T cells are now a possible contributor to Alzheimer’s as well as ALS (the degenerative brain disease amyotrophic lateral sclerosis). Studies in animal models show that without T cells, the inflammation of Alzheimer’s progresses much more rapidly. As T cells protect damaged brains after injury, they may do the same in Alzheimer’s. The same seems to be true of ALS. Studies appear to go along with T cells, peripheral macrophages and microglia protecting against these brain diseases in the early phases (and possibly hurting later).
Brain Injury
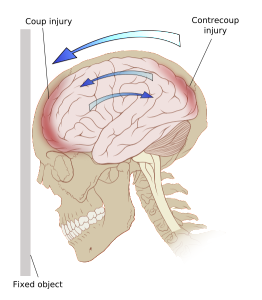
A brain injury produces immune changes throughout the body, not just in the brain. Major areas with inflammation include the injury site, the deep cervical lymph nodes and the cerebrospinal fluid and meningeal spaces. At the site of injury, many cytokines are triggered that produce global efforts in the immune system. These cytokines include interleukin-33 (IL-33), ATP or adenosine triphosphate, alarmin, and HMGB1. Signals trigger glial cells into action and they attract white blood cells, granulocytes and monocytes to help rebuild the damaged site. IL-33 is vital to bring the necessary white blood cells to the region for repair. Brain injury stimulates very high levels of this cytokine compared with other regions of the body, which might also avoid infection from microbes.
Recent research shows that T cells in the meningeal spaces and cerebrospinal fluid are extremely dynamic and changeable. A previous post noted how T cells travel through the CSF and then go out of the brain into the nearby deep cervical lymph nodes. This was only discovered quite recently. Even more recent research shows that they go both ways and affecting one population changes the other. If entry to the nodes is blocked, then the number of T cells entering the CSF doubles. This research is just beginning and the details of the different types of T cells in these spaces related to viral and bacterial infections and to autoimmune diseases are not yet clarified.
Immune Cells Travelling In and Out of the CSF
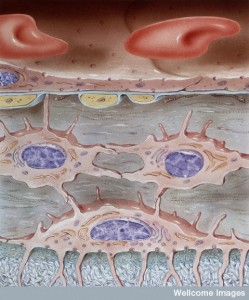 Certainly, inflammation increases the number of immune cells in the CSF. Some forces keep these cells in the brain as well. Previous posts described how signals from choroid cells allow entry from blood vessels to CSF and from pericytes, capillaries, and astrocytes allow entry from blood vessels to the brain tissues. But, the details are just being discovered. A previous post detailed the many signals that pericytes make to astrocytes, capillaries, and neurons.
Certainly, inflammation increases the number of immune cells in the CSF. Some forces keep these cells in the brain as well. Previous posts described how signals from choroid cells allow entry from blood vessels to CSF and from pericytes, capillaries, and astrocytes allow entry from blood vessels to the brain tissues. But, the details are just being discovered. A previous post detailed the many signals that pericytes make to astrocytes, capillaries, and neurons.
Choroid cells cover capillaries and are next to tissue cells in the brain. Choroid cells provide fluid for the CSF through fenestrated membranes with many inter digitations. The blood is filtered to produce CSF by many close contacts to small blood vessels that contain a large number of immune cells. Cells must traverse both the lining cells of blood vessels as well as the choroid cells that are very tightly joined.
To enter through meningeal vessels to the CSF, cells and large molecules must cross a special barrier the blood-meningeal barrier (BMB), which is not as tight as the blood brain barrier (BBB). The BBB includes astrocyte end feet covering blood vessels. Therefore travelling through the BMB is easier for cells.
Recent research shows that meningeal blood vessels actually attract T cells to come into the brain, although the exact signals are not yet known. Once in the meningeal spaces, T cells are activated and then are able to get into tissue. Cells are then able to travel across pia mater to get into brain tissue. When there is inflammation in the brain either from microbe infection or inflammation directly stimulated by neurons often as part of neuroplasticity (neuroinflammation), there is a gradient of cytokines helping with the crossing of the blood brain barrier. In MS, CD8+T cells are helped in this process. They are especially attracted to the brain.
Cleaning the Brain
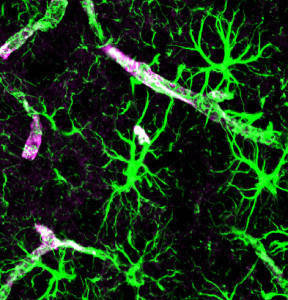
There is even less known about the exact ways that immune cells leave the brain. It appears that newly discovered lymph vessels are a major exit route. Debris is generally cleared through the lymph system in the entire body, but there are no lymph vessels in the brain tissue that have yet been found.
Recently, a new channel that is not lymph was described between the lining cells of the blood vessels. This lining consists of capillaries and pericytes, and end feet of astrocytes that surround all blood vessels and regulate the amount of blood flow to a region of neurons. This space has been called “glia-lymphatic” or “glymphatic”.
Cerebrospinal fluid enters the brain tissue along arteries that are pulsating. This pulsation produces movement of the fluid and the large molecules in the fluid into brain tissue. From this space between neurons and glia it is then absorbed into the space near the outgoing venules. This absorption ocurs by the action of a special type of astrocytes that sits around these venules. This action is based on a water channel molecule called aquaporin 4. Travelling from near the artery to the venules the region in between glia and neurons is cleared of many molecules including misfolded proteins. Surprisingly, this goes back into the CSF.
Also, of note, in this newly discovered glymphatic system, during deep sleep, neurons shrink considerably, possibly 40%, making the liquid space that is being cleared much larger. This is one reason why sleep is so valuable to clean out misfolded proteins and avoid dementias.
 Each day a large amount of CSF fluid is made—equivalent to six times the total amount in all the ventricles. Therefore, most of it drains out every day into special venous sinuses in the arachnoid granulations. But, this draining doesn’t include immune cells.
Each day a large amount of CSF fluid is made—equivalent to six times the total amount in all the ventricles. Therefore, most of it drains out every day into special venous sinuses in the arachnoid granulations. But, this draining doesn’t include immune cells.
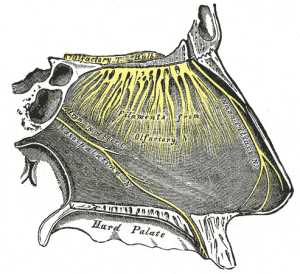
Another route out of the brain is the cribiform plate. This plate is part of the skull and is where olfactory nerves leave the brain and appear in the nose. Travelling along these nerves, immune cells would rapidly place cells near lymph vessels in the nose. This would bring them to vital deep cervical lymph nodes. It is in these lymph nodes that T cells pick up trails of microbes, cancers and damaged cells that have been in the brain.
But, another route has been resurrected from distant past research. It appears that recently identified lymphatic vessels in the meninges could be a major route for both large molecules and immune cells. These were considered in very early research but without proof of their existence, have not been considered again until recently. This is because there is now increased possibility to observe them. There also appear to be lymph vessels in the eye that were not considered either. Research from 40 years ago in MS patients discussed possible lymph drainage. It is conceivable that during inflammation, lymphatic vessels in the meninges extend into the tissue to drain.
Unfortunately, there is no clear way to identify lining cells of these lymph vessels. Current methods also identify other cells at the same time without a way to distinguish them. As of now there is no evidence of lymph vessels in the tissues, only in the meningeal space.
Interrelationship and Evolution of Immune and Brain
 Previous posts showed that many molecules are used for both immune and brain functions. Examples of this are immune globulin molecules that are used for guidance of migrating neurons and glia. Previous posts also show intense signaling occurs between the wired and wireless brain.
Previous posts showed that many molecules are used for both immune and brain functions. Examples of this are immune globulin molecules that are used for guidance of migrating neurons and glia. Previous posts also show intense signaling occurs between the wired and wireless brain.
Both immune and brain systems developed responding to microbes in constant evolution. It has been hypothesized that the “sick feeling” developed as a result of this fight against microbes. A previous post noted that T cells are instrumental in signaling to the brain when the “sick feeling” is indicated, as well as the to continue memory and cognition during acute stress, and decreased cognition with chronic stress.
 The response to social isolation is more complex. When people isolate themselves while ill, this hurts microbes efforts to spread. From the microbe point of view isolation during sickness doesn’t allow for transmission of the illness. Previous posts show that in some animals microbes affect behavior to increase transmission—such as Toxoplasma making mice friendly to cats.
The response to social isolation is more complex. When people isolate themselves while ill, this hurts microbes efforts to spread. From the microbe point of view isolation during sickness doesn’t allow for transmission of the illness. Previous posts show that in some animals microbes affect behavior to increase transmission—such as Toxoplasma making mice friendly to cats.
But, in a human sense larger social isolation creates problems for society. Social isolation from psychological factors has been shown to cause decrease of immune responses to inflammation. Recent research suggests that responses to dangerous microbes such as with the cytokine interferon-γ evolved related to social behavior.
Possible Recent Conclusions
 Meningeal lymphatic vessels have only very recently been discovered and they seem to be a way that T cells and large molecules exit brain tissue and CSF. Getting into the tissue itself involves signals that are not yet fully understood.
Meningeal lymphatic vessels have only very recently been discovered and they seem to be a way that T cells and large molecules exit brain tissue and CSF. Getting into the tissue itself involves signals that are not yet fully understood.
T cells in the meninges have specific responses to brain cell antigens. This can create autoimmune disease. Fluid from between the neurons and glia in brain tissue drains into cerebrospinal fluid. There, immune cells take samples and present them to T cells. In response, T cells promote these same immune cells in the brain with special cytokines. This signaling maintains brain function. When these become a problem, then the immune cells invade brain tissue creating autoimmune disease. It is also possible that in the meninges possess a reservoir of memory T cells. As T cells signal to the brain, these memory cells might be part of the message.
Update on Interface of Immunity and Brain
 As the ability to see tiny interactions among cells, the mechanisms of the wired and wireless brain are being discovered. It is clear that there is constant signaling and communication between all kinds of cells including brain, immune, blood, lining of gut, and microbes. Decisions made in these cellular conversations determine health and disease.
As the ability to see tiny interactions among cells, the mechanisms of the wired and wireless brain are being discovered. It is clear that there is constant signaling and communication between all kinds of cells including brain, immune, blood, lining of gut, and microbes. Decisions made in these cellular conversations determine health and disease.