 The brain uses 20% of the body’s energy, although it weighs three pounds. Humans have more genes operating to metabolize energy for the costly cognitive brain than other species. Recent research is now showing which neuronal activity uses the most energy. In fact, it is the activity of the ion channels along the axon and synapses that uses most of the energy. Astrocytes use much of the rest carefully monitoring and regulating the energy usage.
The brain uses 20% of the body’s energy, although it weighs three pounds. Humans have more genes operating to metabolize energy for the costly cognitive brain than other species. Recent research is now showing which neuronal activity uses the most energy. In fact, it is the activity of the ion channels along the axon and synapses that uses most of the energy. Astrocytes use much of the rest carefully monitoring and regulating the energy usage.
Astrocytes regulate the blood flow in each small brain region and this relationship of neurons and astrocytes is key for all energy use. The fMRI measures this blood flow, not neuronal energy use. It is recent observation of cells that has advanced the field of brain energy usage and allows analysis of the molecules used in imaging.
Brain Cells Use More than Glucose for Energy
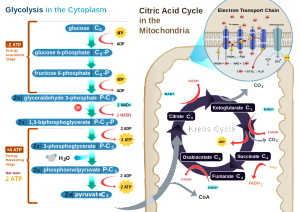
Most of the time glucose is the energy particle used by the brain through an oxidation pathway. But, at times of fasting, newborn diet and diabetes, ketones are used for energy. A surprising amount of the glucose (10%) provides neuronal energy through a process producing lactate (aerobic glycolysis—lactate with oxygen present). This is 30% of the energy during the fetus brain development. In adults, this process only operates in specific regions of the cortex providing 25% of the energy. In other brain areas it doesn’t exist. This process also occurs frequently in astrocytes.
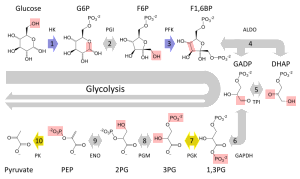
Different types of brain cells have different usage of energy and different metabolism. The most is known about neurons and astrocytes, less about microglia and oligodendrocytes. Neurons are mostly oxidative and astrocytes use mostly glycolysis. Oxidation in mitochondria produces up to 36 high-energy ATP molecules for every glucose molecule, if all of the metabolites are completely processed. This is variable based on what other pathways are operating at the time to produce particular molecules. This well-known complex process includes the tricarboxylic (TCA) cycle consuming oxygen and transferring electrons and makes CO2 and water. Lactate can be produced and this can be transferred to other cells.
 Astrocytes also have mitochondria, but regulate the addition of pyruvate into the TCA, which makes lactate. Signaling pathways using phosphorylation regulates this process. Both in the astrocyte and the neuron, making lactate involves very complex metabolic pathways that have just been discovered. If these pathways don’t work, it can kill the neuron by apoptosis. It shows that a critical function of glucose in neurons is to maintain an anti oxidant condition so it will not die. In astrocytes, there are genetic networks that drive glucose to aerobic glycolysis and lactate.
Astrocytes also have mitochondria, but regulate the addition of pyruvate into the TCA, which makes lactate. Signaling pathways using phosphorylation regulates this process. Both in the astrocyte and the neuron, making lactate involves very complex metabolic pathways that have just been discovered. If these pathways don’t work, it can kill the neuron by apoptosis. It shows that a critical function of glucose in neurons is to maintain an anti oxidant condition so it will not die. In astrocytes, there are genetic networks that drive glucose to aerobic glycolysis and lactate.
Another critical aspect of sugar metabolism is the new field of glycobiology. There are many special sugar attachments to proteins throughout the brain. Some of the end products of glycolysis are AGEs (advanced glycation end products) that are critical in neurodegenerative disease. One is MG or methylglyoxal that his highly regulated and metabolized in astrocytes to avoid destruction of the cell. Astrocytes protect neurons from MG toxicity through these complex metabolic pathways.
Neurons and astrocytes are therefore, complementary. Neurons produce NADPH that can protect against oxidation products to avoid oxidative stress (from high mitochondrial activity). Astrocytes mostly make lactate and pyruvate. Lactate is sent to neurons for more energy production. These two opposite glucose processes in the two different cells are necessary for neuronal survival.
Energy Budget of the Neuron
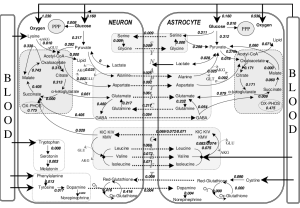 Neurons use up to 80% of the brain energy, with glia using the rest. Much of this is for the ion pumps that produce the axon and synapse electrical potentials. Surprisingly most is used in the synaptic region with the mylenated axons being more energy efficient. The synapse is very distant from the cell body and therefore complex mechanisms exist to monitor this usage.
Neurons use up to 80% of the brain energy, with glia using the rest. Much of this is for the ion pumps that produce the axon and synapse electrical potentials. Surprisingly most is used in the synaptic region with the mylenated axons being more energy efficient. The synapse is very distant from the cell body and therefore complex mechanisms exist to monitor this usage.
A new technique showed the local electrical field potentials (see post on Electricity in the Brain) were correlated with activity at the synapse. This, also, showed that most energy is used at the synapse. It is the astrocytes close monitoring of the synapses that has emerged as the critical factor in regulating the energy.
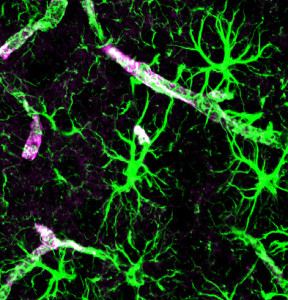
One of the arms of the astrocyte is called the end foot and entirely encircles the capillary wall and others surround the components of the synapse. They release soluble materials into the extracellular space, which drain via the glymphatics and the CSF.
Surrounding the synapse, astrocytes make particular proteins as receptors and uptake sites, especially for glutamate secreted by the neurons. This glutamate uptake triggers lactate. Some of the glutamate is metabolized to glutamine and returned to the neuron. Others go into metabolic cycles. A complex regulation of glutamate transporter molecules feeds the variety of different cycles that produce many different functions. One of these mechanisms using lactate controls the activity of pyramidal neurons nearby. One mechanism is that glutamate causes mitochondria to change their pH and their functions.
 Another aspect of metabolism is that at different phases of synapse activity, there are different types of glucose usage. Oxidative metabolism exists in the early phase, which uses less oxygen altering the fMRI signal of the blood oxygen levels. Then there is a rapid use of lactate from the extracellular space. The complementary process between the neuron and the astrocyte maintains this lactate. The astrocytes ability to respond in complex ways to glutamate is critical for neuroplasticity and is dependent upon this complementary metabolic relationship.
Another aspect of metabolism is that at different phases of synapse activity, there are different types of glucose usage. Oxidative metabolism exists in the early phase, which uses less oxygen altering the fMRI signal of the blood oxygen levels. Then there is a rapid use of lactate from the extracellular space. The complementary process between the neuron and the astrocyte maintains this lactate. The astrocytes ability to respond in complex ways to glutamate is critical for neuroplasticity and is dependent upon this complementary metabolic relationship.
As the astrocyte becomes more capable of glycolysis, metabolism of glycogen and creation of glutamine, genetic networks are triggered. The opposite happens in the neuron where it becomes less capable of these same abilities to metabolize glucose and glutamine.
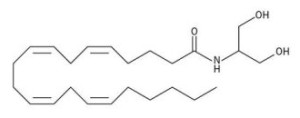 Astrocytes develop the ability for glycolysis and to export lactate in time for activation of the neuron. There are other triggers for this in addition to glutamate uptake—arachidoninic acid (see post on lipoproteins), norepinephrine and potassium. Higher levels of potassium occur outside of the axon during the action potential. These work at different time scales with the potassium being rapid and the glutamate slow over a longer time period.
Astrocytes develop the ability for glycolysis and to export lactate in time for activation of the neuron. There are other triggers for this in addition to glutamate uptake—arachidoninic acid (see post on lipoproteins), norepinephrine and potassium. Higher levels of potassium occur outside of the axon during the action potential. These work at different time scales with the potassium being rapid and the glutamate slow over a longer time period.
Glycogen is the molecule that is used to store glucose in astrocytes. Particular neurotransmitters stimulate breakdown of glycogen—vasoactive intestinal peptide (VIP) and norepinephrine. VIP neurons exist throughout the cortex in special interneuron bipolar cells. The thalamus and cortical circuits stimulate VIP neurons. They have a very large dendrite arbor that encompasses all of the cortex layers and measures energy needs. They send signals to astrocytes to break down glycogen in very particular vertical radial layers and columns of the cortex. The norepinephrine comes from the locus coeruleus and, also, spread across the entire cortex but horizontally, also, signaling for glycogen.
Both the glutamate and the VIP mechanisms release lactate from astrocytes for neurons.
Astrocyte Networks
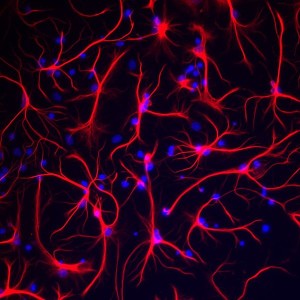
Other posts have described the efforts made to elucidate the vast astrocyte network. This system, while critical for all brain function, has been largely ignored until recently by the neuron centric neuroscience. Astrocytes use electrical gap junctions, which can also regulate metabolites. The astrocyte effect is widespread and can send nutrients to sustain a large number of neurons some at great distance. This system can send lactate to neurons at great distance. A previous post described the recently discovered calcium waves in astrocyte networks. Now, this current research describes metabolic waves. Lactate transmission at gap junctions, also, occurs with astrocytes and oligodendrocytes.
Lactate Use Is Also Vital
 While glucose is the major source of energy in the brain, lactate (made of three carbons) is now seen as vital as well. From the lactate crossing the CSF, 10% of the energy can be sustained. With exercise lactate increases 10 times and the brain uses much more—up to 25%. This is instead of glucose. The mechanism described above where astrocytes produce lactate from glycogen for neurons it is even greater usage.
While glucose is the major source of energy in the brain, lactate (made of three carbons) is now seen as vital as well. From the lactate crossing the CSF, 10% of the energy can be sustained. With exercise lactate increases 10 times and the brain uses much more—up to 25%. This is instead of glucose. The mechanism described above where astrocytes produce lactate from glycogen for neurons it is even greater usage.
Studies show that if given both glucose and lactate, neurons will use lactate. This means less glucose is used. There are number of examples, including the retina, recently discovered where lactate is preferentially used for energy after being made from glucose and used. It is especially useful if there is less blood flow such as stroke or hypoxia. In studies, strokes can be minimized by infusing lactate. When axons do not have enough food, glycogen mobilizes and produces more lactate that is sent to the axons. It is possible that ways the increase the astrocytes ability to make more lactate can help stroke damage. When lactate is not sufficient, there is more neuronal damage.
Previous posts have shown that metabolic cycles produce products that have a double life as signals. They send messages to the genetic networks in the nucleus. (see post on How Do Microbes Make Decisions?). Lactate is a signal in different regions such as the hypothalamus where it signals the amount of glucose and orexin and the cortex where it signals GABA. Breathing rate is regulated by lactate in the medulla where it measures pH.
Lactate from astrocytes is critical for learning and memory. Neuroplastic changes in neurons are dependent on the glycogen transfer from astrocytes. It appears to be mainly for long term memory and is dependent on a specific lactate transporter. Without this transporter amnesia is a result. It is lactate, not glucose, that simulates the plasticity with BDNF.
Astrocytes and Imaging
 fMRI measures the difference between hemoglobin that has oxygen and that doesn’t. It, also, reflects changes in blood flow (blood oxygen level dependent contrast or BOLD). Studies using electrical measurements finds this closest to the local field potential rather than spikes. It appears to measure afferent inputs activity at synapses.
fMRI measures the difference between hemoglobin that has oxygen and that doesn’t. It, also, reflects changes in blood flow (blood oxygen level dependent contrast or BOLD). Studies using electrical measurements finds this closest to the local field potential rather than spikes. It appears to measure afferent inputs activity at synapses.
Energy is particularly used by the mechanism of vesicles at the synapse that releases neurotransmitters. This is mainly glutamate that is picked up as the signal of increased activity in the synapse. If the signaling pathways in the astrocyte are affected, then this will show on the fMRI. So, in fact, it measures a combination of activity in the neuron and the astrocyte. One issue is that the glucose use and oxygen use are not the same. Glucose increases occur at the same time that venus capillaries have oxygen still there with less deoxyhemoglobin. The magnetic properties of oxy and deoxy are different showing activity.
Glutamate picked up by astrocytes increases uptake of glucose processed by aerobic glycolysis, making lactate. Oxygen doesn’t increase as a result. Therefore, fMRI signals at first go down when there is activity, then a longer increase. PET scan can pick up metabolic or vascular differences, including glucose use and oxygen consumption.
Energy Use and Human Brain Evolution
 Different species have very different uses of brain energy. Humans use 10 times as much energy as non-human primates and 100 times more than mice. The percentage changes, also. Humans use twice the percentage as non-human primates and 10 times more than mice.
Different species have very different uses of brain energy. Humans use 10 times as much energy as non-human primates and 100 times more than mice. The percentage changes, also. Humans use twice the percentage as non-human primates and 10 times more than mice.
This increase is greater than the body proportions. The very large unique human neocortex uses much of the energy. The cortex has 16 billion neurons, whereas non-human primates have 5 billion and mice 30 million. The complexity of axons and dendrites increases even more. Glucose use per unit size is not greater because each neuron takes up so much more space. It is not yet clear if the increased arbors of dendrites are the main factor in increasing energy use. There are a greater amount of astrocytes in humans than other species and there are more astrocytes than neurons. Astrocytes predominantly take up glucose.
Each neuron uses up three times the ATP to maintain the electrical potential and to fire. There are a much greater amount of genes for energy production in humans. The production of ATP by oxidation has the most gene networks. This is the most efficient pathway, but, also, produces oxidative products that are dangerous to the neuron and have to be controlled. There are four times as many new metabolic pathways in the human cortex than other species. One reason the body of the fetus is developed slowly is that the brain uses so much of the energy. It, also, has special enzymes to use the lactate as the main source of neuron energy.
Regulation of Brain Energy
 As with everything in the brain, the way energy is used efficiently is complex. There are more metabolic energy pathways that have been recently discovered. The brain uses 20% of all the body’s energy in humans. Neurons use 80% of this for the electrical activity near the synapses. The myelinated axons are the most efficient. Astrocytes use much of the rest of the energy.
As with everything in the brain, the way energy is used efficiently is complex. There are more metabolic energy pathways that have been recently discovered. The brain uses 20% of all the body’s energy in humans. Neurons use 80% of this for the electrical activity near the synapses. The myelinated axons are the most efficient. Astrocytes use much of the rest of the energy.
It is the astrocytes that regulate the synapses and energy usage. They also regulate the blood flow that brings material used to burn for energy. The communication between the neuron and astrocyte is vital to maintain this critical energy balance for survival of the brain.