 Recent posts had already shown that gut microbe signaling with the human brain can have positive and negative effects on anxiety, stress, depression, obesity and degenerative illness. This occurs by neurotransmitters secreted into the blood, gut neuron stimulation, microbe travel into the brain, and immune cells manipulation. Other posts show tricks bacteria and viruses use to enter and travel in neurons, even commandeering transport machinery along axon microtubules.
Recent posts had already shown that gut microbe signaling with the human brain can have positive and negative effects on anxiety, stress, depression, obesity and degenerative illness. This occurs by neurotransmitters secreted into the blood, gut neuron stimulation, microbe travel into the brain, and immune cells manipulation. Other posts show tricks bacteria and viruses use to enter and travel in neurons, even commandeering transport machinery along axon microtubules.
This post provides update of the most recent research.
Many Kinds of Signals in the Developing Brain
 Many different kinds of signals produce the development of the brain during the fetus. A previous post shows the quite amazing process that builds the human brain. Recent research finds a surprising number of different kinds of signals from outside of the brain in the environment are needed as well.
Many different kinds of signals produce the development of the brain during the fetus. A previous post shows the quite amazing process that builds the human brain. Recent research finds a surprising number of different kinds of signals from outside of the brain in the environment are needed as well.
One source of these signals is microbes living in the gut that produce a large amount of products. A previous post showed that there is 300 times the amount of DNA in the resident microbes compared with the human genes. We are just beginning to learn about these products and their effects. But, it is now known that they can be influential in the communication between the gut and the brain in normal cognition, but also depression, anxiety, schizophrenia, Parkinson’s, and autism. In fact, microbe communication is vital to create the blood brain barrier, to produce new neurons, to build myelin in all brain regions, and to stimulate the behavior of microglia. This gut microbe communication with the brain is, also, significant in animal behavior.
As the previous post noted, building the brain starts in the fetus but continues at least until young adulthood. Another post showed that neuroplasticity changes occur throughout life, with perhaps the best connected brains in intelligent elderly (see post) who do not have degenerative brain disease.
 Much of the brain development occurs with neurons and other cells travelling long distances from where they are produced to where they become active in specific circuits. These processes are very responsive to signals from the environment (experiences). The gut is the place where the greatest amount of molecules are produced in the human body and has by far the greatest amount of microbes—more microbe cells in the gut than the entire human body. This can occur because each microbe cell is much smaller than the human cells. Food particles are very significant molecules in this process, and stimulate changes in the developing brain. Microbes can digest and alter these food particular. Microbe products are also very important in helping the development.
Much of the brain development occurs with neurons and other cells travelling long distances from where they are produced to where they become active in specific circuits. These processes are very responsive to signals from the environment (experiences). The gut is the place where the greatest amount of molecules are produced in the human body and has by far the greatest amount of microbes—more microbe cells in the gut than the entire human body. This can occur because each microbe cell is much smaller than the human cells. Food particles are very significant molecules in this process, and stimulate changes in the developing brain. Microbes can digest and alter these food particular. Microbe products are also very important in helping the development.
Microbes are particularly significant in the production of the human immune system. Production of the many immune cells require different microbes and are important in diabetes, asthma, and inflammatory bowel disease.
Mice raised without germs have alterations in their behavior including excessive activity and self-harm along with problems with memory. These mice have alterations in receptors for serotonin and glutamate and alterations in the vital BDNF factor the helps produce new neurons. They have faulty blood brain barriers, and less myelin in the cortex.
Microbe Signals in the Fetus
 In the third week of development, stem cells that produce neurons are born. The previous post described that later the cortex is developed in humans with stem cells called radial glial cells that occur in the ventricular zone. New cells travel a long distance to the cortical plate, while the stem cells remain. When there are enough cortex neurons at the half way point of the life of the fetus, the glia cells develop later and mostly after birth. The unique structure of the brain from front to back and top to bottom develops through gradients like other bodily organs.
In the third week of development, stem cells that produce neurons are born. The previous post described that later the cortex is developed in humans with stem cells called radial glial cells that occur in the ventricular zone. New cells travel a long distance to the cortical plate, while the stem cells remain. When there are enough cortex neurons at the half way point of the life of the fetus, the glia cells develop later and mostly after birth. The unique structure of the brain from front to back and top to bottom develops through gradients like other bodily organs.
The brain is overbuilt so that experience can mold it. Near birth more than half of the neurons die and those in active circuits remain. These need support from signals that nourish neurons like BDNF. One view is that the more support factors, the more connections and the greater survival.
In mice without microbes, there are greater numbers of neurons produced in the hippocampus and the amygdala. Microbes are necessary in the fetus to help prune the brain to a workable size. Some of the glia come from cell lines that also produce immune cells.
Very recent research questions exactly which immune cells from the yolk sac becomes microglia that enter the brain on the 8th day in the development. There are many microglia by 28 days. Without microbes, the microglia do not develop properly and have unusual shapes. They do not respond well to virus attacks. But, if they are given specific short chain fatty acids (SCFAs – see post on their wide ranging significance as signals) they respond better.
 Without microbes, the blood brain barrier doesn’t develop properly having more holes that allow large molecules in. When microbes were added to germ free mice, the barrier improved its function.
Without microbes, the blood brain barrier doesn’t develop properly having more holes that allow large molecules in. When microbes were added to germ free mice, the barrier improved its function.
Microbe signals affect this in multiple ways. They affect the newly discovered lymph system that goes from the cerebrospinal fluid to subarachnoid space to deep cervical lymph nodes. This system allows immune cells and large molecules to enter and leave the brain. Microbes send hormones and other molecules to the brain. Microbe signals affect the vagus nerve from the gut which has effects throughout the body.
Mother’s Gut and Vagina During Pregnancy
 Microbes in the mother are different during pregnancy and change throughout pregnancy. Late in pregnancy Proteobacteria and Actinobacteria grow, but the most prevalent is Lactobacillus. Just before birth there is much greater diversity in the mother’s vagina. Recent research alters the impression that there are no microbes in the fetus. There are now questions as to whether there are some microbes in the placenta and the amniotic fluid, but their significance is not clear.
Microbes in the mother are different during pregnancy and change throughout pregnancy. Late in pregnancy Proteobacteria and Actinobacteria grow, but the most prevalent is Lactobacillus. Just before birth there is much greater diversity in the mother’s vagina. Recent research alters the impression that there are no microbes in the fetus. There are now questions as to whether there are some microbes in the placenta and the amniotic fluid, but their significance is not clear.
Antibiotics during pregnancy in mice and humans affect the immunity of the baby. These alter gut microbes and in mice, babies showed anxiety and alterations in movement behavior. Mother’s diet changed microbes and produced changes in the baby. High fat diet in obese mice produced children with less social ability and repetitive behavior and less Lactobacillus. When given Lactobacillus, the behavior improved. Antibiotic mice also improved.
Microbes produce molecules in the mother including metabolites, LPS, peptidoglycans (PG), and fermentation molecules. One experiment showed how sterile mice given E. coli in the mother’s gut sent microbe products that reach the fetus and affect development. Material from the bacteria cell wall affect the fetus brain causing an increased number of neurons. This occurs through FOXG1 which regulates new neurons in the brain. Peptidoglycan signals alter cognitive ability. Others change behavior. LPS caused anxiety. Studies showed that the effects are in the fetus not the mother.
 A previous post (Maternal Immune Activation or MIA) showed that when the mother’s immune system is activated, it can alter the baby. Infections during pregnancy can increase risk for schizophrenia and other mental conditions. These effects have been produced also by immune signals that affect specific immune receptors. Also, MIA causes alterations in the microbes of the baby. Giving Bacteriodes fragilis seemed to improve effects from MIA by making intestinal cells less permeable and decreasing dangerous molecules. Also, gut bacteria help produce a special T helper cell (Th17) and a cytokine IL-17A, which are critical for MIA effects.
A previous post (Maternal Immune Activation or MIA) showed that when the mother’s immune system is activated, it can alter the baby. Infections during pregnancy can increase risk for schizophrenia and other mental conditions. These effects have been produced also by immune signals that affect specific immune receptors. Also, MIA causes alterations in the microbes of the baby. Giving Bacteriodes fragilis seemed to improve effects from MIA by making intestinal cells less permeable and decreasing dangerous molecules. Also, gut bacteria help produce a special T helper cell (Th17) and a cytokine IL-17A, which are critical for MIA effects.
Baby’s Brain Development
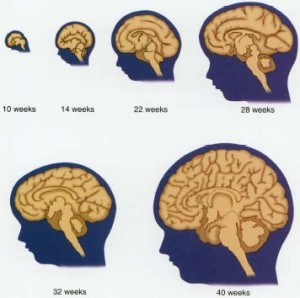 Most brain changes in the infant are producing circuits with synapses and strengthening them through neuroplasticity. This period also prunes many of the circuits that are not used with experience. Production of new neurons slows greatly and occurs only in several vital areas of hippocampus and sub ventricular zone. Glial cells increase greatly. With many new oligodendrocytes, myelin production spreads and continues for thirty years or more in the frontal lobes of the cortex.
Most brain changes in the infant are producing circuits with synapses and strengthening them through neuroplasticity. This period also prunes many of the circuits that are not used with experience. Production of new neurons slows greatly and occurs only in several vital areas of hippocampus and sub ventricular zone. Glial cells increase greatly. With many new oligodendrocytes, myelin production spreads and continues for thirty years or more in the frontal lobes of the cortex.
Antibiotics if given for a long time can decrease the production of new neurons in the hippocampus in mice and affects cognition. There are less particular monocytes, but microglia numbers were normal. These problems decrease with probiotics and exercise. Also, particular monocytes, also, brought back the production of neurons. These imply that making new neurons and pruning neurons and synapses are affected or regulated by microbe signals. Microbes can produce serotonin that will stimulate new neurons.
Astrocytes and microglia prune the synapses in the young baby’s brain. They both produce immune complement molecules—microglia especially produce C1q and make special complement receptors. These are involved in the process of sculpting synapses and circuits in the young baby.
Microbes are needed to produce proper myelination of neurons. With antibiotics, myelin did not occur in some regions of the brain; it did occur in the prefrontal cortex. Microbes are needed as babies to have the proper microbes later in life for a normal brain. One particular immune signal cytokine (CXCL1) and receptors for this signal are necessary to stimulate oligodendrocytes to make proper myelin and this is dependent on having specific microbes.
Baby’s Gut Microbes
 If the fetus has no microbes, then their first exposure is from the mother with Lactobacillus and Prevotella in the vagina. Stress during pregnancy can alter the microbes in the vagina and in the baby. Those from cesarean section have the skin microbes Staphlococcus and Corynebacterium. There are more autoimmune diseases in those born with C-sections. By giving these babies samples of mother’s vagina microbes this can be helped.
If the fetus has no microbes, then their first exposure is from the mother with Lactobacillus and Prevotella in the vagina. Stress during pregnancy can alter the microbes in the vagina and in the baby. Those from cesarean section have the skin microbes Staphlococcus and Corynebacterium. There are more autoimmune diseases in those born with C-sections. By giving these babies samples of mother’s vagina microbes this can be helped.
Microbes in babies are very responsive to antibiotics and different foods that attract various microbes. New environments, especially close contact with siblings, also, affect the type of microbes. Probiotics during pregnancy can alter them as well.
Early on the gut of the infant up to age one, there is no oxygen and there exist anaerobic microbes such as Bifdobacterium, Enterococcus, E. Shigella, Streptococcus, Bacteriodes, and Rothia. This is similar to the mother. By one year of age, they have many other microbes as well such as Clostridium, Roseburia, Eubacterium, Veilonella and others. There are a lot of changes until age 3.
 Very dramatic changes in diet, antibiotics, and lifestyle have effects on which microbes are present and what products they made. Some researchers think that antibiotics beforehand can cause depression and behavior problems later. Some form of this occurs in animals, but only after a long course of antibiotics. One study with vancomycin produced sensory neurological changes in rats when adults. In adult mice, days caused anxiety but only temporarily (two weeks). When broad spectrum antibiotics were given to mice for a long time from childhood to adulthood, behavior and brain molecules were altered.
Very dramatic changes in diet, antibiotics, and lifestyle have effects on which microbes are present and what products they made. Some researchers think that antibiotics beforehand can cause depression and behavior problems later. Some form of this occurs in animals, but only after a long course of antibiotics. One study with vancomycin produced sensory neurological changes in rats when adults. In adult mice, days caused anxiety but only temporarily (two weeks). When broad spectrum antibiotics were given to mice for a long time from childhood to adulthood, behavior and brain molecules were altered.
Probiotics change results of animal experiments. They decreased anxiety, depression type behavior, repetitive behavior and increased social function. They also increased memory and cognition.
Also, in normal humans, probiotics seemed to affect fMRI tests when exposed to faces with emotions.
There appears to be both signaling from microbes to the brain and from the brain to the microbes. One example was stress in animals that caused dramatic changes in the makeup of the microbes in the gut.
Adult Gut Microbes
 Adults tend to have largely stable diverse microbe populations over years. Some factors that determine the type are diet, location, lifestyle and genes. A previous post showed how completely different regions in the gut are. They are different from the mouth, stomach, through small and large intestines, but also in the center of the lumen flow, near the borders of intestinal lining cells, and deep in the crypts. Each forms distinct niches with different populations (see previous post). There are different niches in the skin and vagina. In general, the more diverse the better and correlates with health (or absence of disease). Even with interruptions, they go back to the status quo.
Adults tend to have largely stable diverse microbe populations over years. Some factors that determine the type are diet, location, lifestyle and genes. A previous post showed how completely different regions in the gut are. They are different from the mouth, stomach, through small and large intestines, but also in the center of the lumen flow, near the borders of intestinal lining cells, and deep in the crypts. Each forms distinct niches with different populations (see previous post). There are different niches in the skin and vagina. In general, the more diverse the better and correlates with health (or absence of disease). Even with interruptions, they go back to the status quo.
Brain Disorders and Aging
 Brain disease is often associated with gut problems and food allergies. It was already mentioned above that mice have increased anxiety and stress related to specific microbes and their products. For many years there has been a correlation of stress related illness and gut symptoms, but the cause has not been clear.
Brain disease is often associated with gut problems and food allergies. It was already mentioned above that mice have increased anxiety and stress related to specific microbes and their products. For many years there has been a correlation of stress related illness and gut symptoms, but the cause has not been clear.
With autism, the gut can have increased amounts of diverse species of Clostridia and groups called anaerobes and micro aerobes. Gut symptoms are increased in children with autism and these have altered microbes. One microbe Sutterella was found in this group. Large scale studies with particular microbe replacement with probiotics are just beginning.
There are very few studies involving Schizophrenia. One study showed more lactic-acid bacteria and phage virus associated with this bacteria. Another study showed particular microbes in parents of schizophrenic children. There is not enough research yet for any conclusions.
 Twenty percent of those with depression have gut symptoms. A previous post noted a subset of depression associated with immune changes. One group has less functional microglia in the brain. In mice with depressive symptoms, antibiotic minocycline reduces activity of microglia and decreases depression symptoms. Minocycline has also helped some humans with depression. The exact mechanism is not clear. Depression symptoms in mice correlated with less microbe diversity and fecal transplantation helped reduce the symptoms.
Twenty percent of those with depression have gut symptoms. A previous post noted a subset of depression associated with immune changes. One group has less functional microglia in the brain. In mice with depressive symptoms, antibiotic minocycline reduces activity of microglia and decreases depression symptoms. Minocycline has also helped some humans with depression. The exact mechanism is not clear. Depression symptoms in mice correlated with less microbe diversity and fecal transplantation helped reduce the symptoms.
Aging decreases gut neuronal function and there are frequent gut symptoms in elderly. There is loss of cholinergic neurons and glial cells in the gut plexus, which contribute to less bowel control. Elderly have different microbes—more Bacteroides and less Firmicutes. They are less diverse and are altered by environments. Those in medical institutions have microbes that are very different than other adults. A probiotic with Lactobacillus rhamnosus caused less inflammation with more short chain fatty acids. While microbes change with age, they are unique in those live past a hundred.
Only recently have studies of gut microbes begun in Alzheimer’s and other neurodegenerative diseases. Those with Parkinson’s have very different microbes—increased Lactobacillaceae and decrease of Prevotellaceae. Those with more tremor have lower Enterobacteraceae than the types with gait instability. They have more E. coli.
 Neuro inflammation is just now being researched as related to causes of dementia. Specific immune cytokine signals can cause neuronal death. SCFAs are needed for healthy microglia. There are less SCFAs in Alzheimer patients’ feces. One new result raises the question as to whether amyloid protects the brain by killing microbes.
Neuro inflammation is just now being researched as related to causes of dementia. Specific immune cytokine signals can cause neuronal death. SCFAs are needed for healthy microglia. There are less SCFAs in Alzheimer patients’ feces. One new result raises the question as to whether amyloid protects the brain by killing microbes.
Recently, one rare disease related microbes in the gut and brain deterioration. Microbes stimulate a particular T cell that interacts with the eye and causes inflammation of the middle layer of the eye which destroys neurons in the eye causing lack of vision. Also, these T cells react to molecules in the eye (autoimmune reaction) as well molecules from gut microbes.
Update on Microbes Affecting the Brain
 Brains develop from a wide range of different signals from many different cells in the developing brain, from products that are produced by human cells and microbes in the gut, and, also, from the outside environment, There Some neurological circuits appear to have evolved along with microbes and their products. Others don’t listen to microbe signals, but rather other environmental factors.
Brains develop from a wide range of different signals from many different cells in the developing brain, from products that are produced by human cells and microbes in the gut, and, also, from the outside environment, There Some neurological circuits appear to have evolved along with microbes and their products. Others don’t listen to microbe signals, but rather other environmental factors.
Microbes in the gut (where most live) signal to the brain with their products sent into the blood, by stimulating nerves from the gut such as the vagus, and by traveling in neurons to the brain. Increasing evidence supports the role of microbes in the development of the normal human brain.
There is much to learn in this fascinating relationship of gut microbes and the human brain.