 Until recently all circadian clock rhythms were assumed to be triggered from a central brain clock, synchronizing sleep, wakefulness, hormones, and metabolism. Now, many more functions have been found related to clocks and the variations throughout the body cannot all be triggered by one central clock. In fact, each tissue and organ appears to have unique 24 hour cycles related to the specific functions of the organ and the unique metabolic functions. Also, cycles of available material and cellular activity appears to ubiquitous.
Until recently all circadian clock rhythms were assumed to be triggered from a central brain clock, synchronizing sleep, wakefulness, hormones, and metabolism. Now, many more functions have been found related to clocks and the variations throughout the body cannot all be triggered by one central clock. In fact, each tissue and organ appears to have unique 24 hour cycles related to the specific functions of the organ and the unique metabolic functions. Also, cycles of available material and cellular activity appears to ubiquitous.
The previous post described the unusual and unexpected finding that all cells have their own individual clock. It now appears that individual cells have a basic core genetic clock and then a wide range of ancillary oscillations with genetic loops that synchronize the unique metabolism and behavior of each cell, each tissue, and each organ. How all of these loops and cycles relate to each other and to the central clock that sends out neurological messages and hormones in a 24 cycle is just now being discovered. This post discusses the latest findings about metabolism, individual clocks, tissue clocks, and central clocks. The next post discusses unique clocks affecting immune function, and then a post will discuss brain clocks. See the previous post for background and an introduction to the concept that each cell has a clock. This is another layer of specific communication among all cells.
 A large number of genetic networks with feedback loops are part of the core clock in each cell. These are determined by levels of factors interacting with specific genes that are activated and repressed by protein transcription factors. Also, feedback loops use variations in the processes that make proteins from messenger RNA. Yet, another layer of regulation and feedback loops comes from the many cycling epigenetic tags that are placed on the histone spools that protect particular genes. The tags determine whether the spool opens to allow the use of that particular local DNA. Another layer of regulations are modifications of the proteins that serve as transcription factors to start and stop genes.
A large number of genetic networks with feedback loops are part of the core clock in each cell. These are determined by levels of factors interacting with specific genes that are activated and repressed by protein transcription factors. Also, feedback loops use variations in the processes that make proteins from messenger RNA. Yet, another layer of regulation and feedback loops comes from the many cycling epigenetic tags that are placed on the histone spools that protect particular genes. The tags determine whether the spool opens to allow the use of that particular local DNA. Another layer of regulations are modifications of the proteins that serve as transcription factors to start and stop genes.
Loops making up the individual cellular clock are extremely complex. But, perhaps even more complex are the differences in associated loops and cycles that are specific for each different type of tissue and each type of cell. The associated loops coordinate with the core clock in many different ways that are just being discovered.
This post will discuss how metabolic processes are coordinated by interactions with the individual cellular clocks in specific organs. There is much that is not yet known about this critical research.
Metabolic Clocks in Human Cells
Human cells (as well as most other animals), have a large number of genes (thousands) that function in loops defining a 24-hour clock. This is one of the largest set of genetic networks influencing the most significant functions of physiology.
These networks are partially influenced by light of day and dark of night, but also times of eating and sleeping. These clock changes affect conversations among cells and tissues where they reside. Genetic and epigenetic networks produce specific times to perform particular functions that would be hard or impossible to do at the same time. These optimize use of energy for the animal as a whole. Disrupting these processes can lead to diseases of metabolism. Recent research shows that rigid adherence to feeding and fasting can impact these abnormal rhythms and might be able to treat chronic disease.
One example of an important metabolic clock regulates blood sugar levels that increase after eating. If someone eats the same amount of food for breakfast, lunch, and dinner, the blood sugar is lowest after breakfast and the greatest after the dinner meal. This is because the clock stimulates melatonin in the evening that interferences with insulin’s effect. As melatonin wears off through the night, the blood level is highest at night and lowest before breakfast (dawn). This also has effects based on the amount of sugar taken up by the brain’s central clock.
 In human cells (as well as most other animals), thousands of genes in loops define a 24-hour clock. This is one of the largest genetic networks influencing the largest regulation of physiology. These networks can be partially influenced by light of day and dark of night, but also times of eating and sleeping.
In human cells (as well as most other animals), thousands of genes in loops define a 24-hour clock. This is one of the largest genetic networks influencing the largest regulation of physiology. These networks can be partially influenced by light of day and dark of night, but also times of eating and sleeping.
These clock changes are connecting with conversations between the cells and the tissues where they reside. Genetic networks produce specific times to perform particular functions that would be hard or impossible to do at the same time. These also optimize the use of energy for the animal as a whole. Disruption can lead to diseases of metabolism. Recent research shows that rigid adherence to feeding and fasting can impact these abnormal rhythms and possibly treat chronic disease.
Regulation of Cellular Clock
 The clock is not easy to define. Clock rhythms were defined as changes that occur unrelated to any signals from the environment. But, in fact this is generally true at the cellular level, but is complicated by the fact that rhythms are affected over time by patterns of behavior and external cues such as food intake and light. Eating high energy foods multiple times per day and disruption from aging can alter rhythms and create disease. Clocks can be disrupted by dramatically altered by different exposures to light over time.
The clock is not easy to define. Clock rhythms were defined as changes that occur unrelated to any signals from the environment. But, in fact this is generally true at the cellular level, but is complicated by the fact that rhythms are affected over time by patterns of behavior and external cues such as food intake and light. Eating high energy foods multiple times per day and disruption from aging can alter rhythms and create disease. Clocks can be disrupted by dramatically altered by different exposures to light over time.
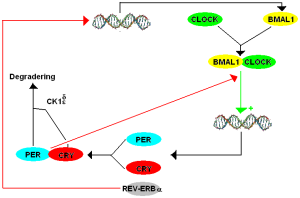
The network of genes that create the timed loops producing the internal clock of the cell has a strange and hard to remember name. It is called TTFL for transcriptional-translational feedback loop. Transcription, we remember, is the process of making messenger RNA from pieces of the DNA code. Then translation is where a protein is made from a piece of messenger RNA in the ribosome. This protein can then act upon the DNA or RNA creating a positive or negative feedback loop.
Proteins that affect DNA production of RNA are called transcription factors. These bind to regions just before the gene as promotors—to start the production of RNA. Another place will stop the process—repressors.
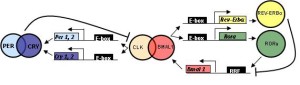 CLOCK (also called NPAS2) and BMAL1 are proteins that land in places to start and to stop the genetic clock cycle. These are basic elements of the core genetic clock loops in every cell. The latter can occur by the protein stimulating (landing on promotor) of a repressor protein that stops the cycle. The repressor is called cyrptochrome or Cryl. Several other proteins (REV-ERB and ROR) are vital to the regulatory feedback loops.
CLOCK (also called NPAS2) and BMAL1 are proteins that land in places to start and to stop the genetic clock cycle. These are basic elements of the core genetic clock loops in every cell. The latter can occur by the protein stimulating (landing on promotor) of a repressor protein that stops the cycle. The repressor is called cyrptochrome or Cryl. Several other proteins (REV-ERB and ROR) are vital to the regulatory feedback loops.
There are many different molecules both in the nucleus and the cytoplasm that make this process extremely complex and hard to describe without extensive jargon. Thirteen vital regulator molecules are triggered by many factors and loops producing 24-hour cycles of the levels of the messenger RNA and the proteins themselves.
These same regulators affect thousands of genes that can produce many effective molecules in exact interrelated rhythms. Timing is achieved by using both RNA and proteins in patterns that would be impossible, if only proteins or RNAs were used alone.
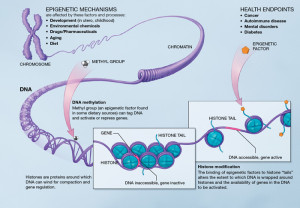 There is more complexity than just loops related to proteins and RNAs. Epigenetic tags on the DNA and on the histone spools of the DNA are integral parts of both the core clock and interacting gene networks for specific cells and tissues. Many different enzymes are affected by histone tags, and others create new tags or alter other tags.
There is more complexity than just loops related to proteins and RNAs. Epigenetic tags on the DNA and on the histone spools of the DNA are integral parts of both the core clock and interacting gene networks for specific cells and tissues. Many different enzymes are affected by histone tags, and others create new tags or alter other tags.
All of this tagging and un tagging determines which parts of DNA are used and therefore which RNA and proteins are produced. In one of many examples CLOCK and BMAL1 proteins are correlated with a particular tagging enzyme (histone acetyl transferase p300 and another CREB-binding protein – CBP). There are many others. Such as repressors of HDACs (histone deacetylase enzymes), a lysine demethylase enzyme (JARID1a), in addition to many other proteins. All of these are involved in creating cycles. Sometimes tags such as adding and subtracting an acetyl tag to histones make a cycle, but tags on proteins can also be the basis of other cycles.
Metabolic Regulation and Cellular Clocks
 With so many moving parts of each cycle, different signals from the outside of the cell can trigger pathways to interact with the cycles. These pathways connect physiological functions and the clock in both directions. Outside factors can be from temperature, hormones, food, etc. One example (out of thousands) is the molecule that carries oxygen in the blood—heme. For heme metabolism, cycles are produced by many different well-known factors in metabolic pathway loops (NAD/NADH, NADP/NADPH, AMP/ATP, Acetyl CoA, SAM, and many others). These alter the vital molecules in the genetic clock cycle. These alter histone modifications, alter proteins, change interactions of proteins, change interactions of proteins that land on DNA sections, and the levels of production of messenger RNA and proteins.
With so many moving parts of each cycle, different signals from the outside of the cell can trigger pathways to interact with the cycles. These pathways connect physiological functions and the clock in both directions. Outside factors can be from temperature, hormones, food, etc. One example (out of thousands) is the molecule that carries oxygen in the blood—heme. For heme metabolism, cycles are produced by many different well-known factors in metabolic pathway loops (NAD/NADH, NADP/NADPH, AMP/ATP, Acetyl CoA, SAM, and many others). These alter the vital molecules in the genetic clock cycle. These alter histone modifications, alter proteins, change interactions of proteins, change interactions of proteins that land on DNA sections, and the levels of production of messenger RNA and proteins.
The clock of an individual cell, therefore, is made up of the genetic network that is unrelated to anything else in the metabolism pathways or signals from outside of the cell. Given this structure, a remarkable finding is that the cells can use these cyclic mechanisms to predict future needs and alter the clocks. This has been observed by microbes in the ocean, plants, and humans.
Proteins inside of the clock mechanism can alter rhythms of proteins outside of the cycle. One example is the circadian proteins REV and CRY affecting hormone receptors that produce regulators of sugar metabolism and are part of story of diabetes. This same REV is involved in altering lipid metabolism as well. This process involved CLOCK/BMAL among others.
This later process only occurs in liver cells, not other types of cells. Some other processes only occur in brain cells. The ways that specific tissues affect their clocks in unique ways is being researched.
Central Clocks Talk to Tissues
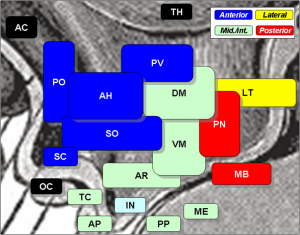 The central clock in the brain is in the hypothalamus and is called the suprachiasmatic nucleus or SCN. The hypothalamus has many centers that direct physiological parameters. For a long time the SCN was considered the only clock with neurons responding to sleep/wake and feeding cycles of the animal. SCN signals oscillate and affect other brain cells and hormone production. It produces a cycle that stimulates ACTH adrenocorticotropic hormone in the in the pituitary that then produces cortisone steroids in the adrenal related to the time of day and night.
The central clock in the brain is in the hypothalamus and is called the suprachiasmatic nucleus or SCN. The hypothalamus has many centers that direct physiological parameters. For a long time the SCN was considered the only clock with neurons responding to sleep/wake and feeding cycles of the animal. SCN signals oscillate and affect other brain cells and hormone production. It produces a cycle that stimulates ACTH adrenocorticotropic hormone in the in the pituitary that then produces cortisone steroids in the adrenal related to the time of day and night.
Steroids triggered in this cycle have vast metabolic effects throughout the body. They help us wake up and trigger muscles to get energy. This is all connected to the melatonin cycle. It is closely connected with centers for hunger, sleep, temperature, and blood concentration. It has conversations with the gut and other tissues that all have their own clocks as well. These clocks are coordinated related to the time to take in more sugar and the release of insulin and other hormones (glucagon, etc.) Therefore, the SCN’s cycles and genetic loops direct melatonin, steroids, growth hormone, insulin, glucagon, and others. Major effects occur in the liver.
In the Liver
 The liver is the central organ that deals with vast metabolic functions and is a major site of cellular clock research. Its clocks are very significant and complex. Research is somewhat easier than elsewhere since most cells are the same type and all are very connected to communication through blood factors.
The liver is the central organ that deals with vast metabolic functions and is a major site of cellular clock research. Its clocks are very significant and complex. Research is somewhat easier than elsewhere since most cells are the same type and all are very connected to communication through blood factors.
One set of mouse studies of the liver places them in darkness. This shows natural clocks for one big feeding and multiple small feedings. This pattern appears to be more energy efficient. When there is lack of food, this cycling is reduced. Having specific cycles allows mice to separate metabolic processes that are not compatible, such as making and destroying the same molecules. Several significant enzymes work in both the metabolic cycle and the clock cycle.
The liver is vital for energy regulation; it stores food during feeding, and brings it out when needed for fasting. Because eating occurs during waking these cycles are highly connected.
Glucose is taken into the body and then stored in liver cells where it is tagged with phosphate energy molecules. It then becomes part of the cycle making energy (glycolysis). It is stored as glycogen triggered by insulin that later produces glucose when needed. Like the heme example above, there are a large number of molecules and pathways in these feedback loops and they are connected with clock molecules CLOCK/BMAL1. These pathways are also connected to production of other major molecules such as amino acids and nucleotides. All of these cycles are connected with the clock in extremely complex ways. One of the clock proteins is a coordinating point between current responses and future planning of responses related to energy needs.
 Fatty acids are highly regulated in the liver. An enzyme in the citric acid cycle that limits the speed of the cycle is also part of clock pathways. These produce a daily cycle producing fatty acids during eating and the opposite oxidation pathway when not eating. Many of the molecules in the fatty acid synthesis are on the clock cycle.
Fatty acids are highly regulated in the liver. An enzyme in the citric acid cycle that limits the speed of the cycle is also part of clock pathways. These produce a daily cycle producing fatty acids during eating and the opposite oxidation pathway when not eating. Many of the molecules in the fatty acid synthesis are on the clock cycle.
Proteins are eaten, then broken into amino acids in the small intestine. Amino acids are sent to the liver cells and used to make proteins, to make energy when needed, to be used for parts of other molecules, and altered to ammonia for the urea cycle. These processes are coordinated with mTOR (see post) in very complex loops and pathways like the heme example above. The post on mTOR notes how through signaling it regulates the use of ribosomes and the need for proteins. The liver makes many vital proteins, such as albumin and those used in complement immune functions. These are also coordinated with use of proteins in muscle as well. The cycles for most amino acid activity are greatest at night when fasting. This includes total amino acids in the blood, branched amino acid production, and urea.
Small Molecules in Metabolism Are Clock Signals
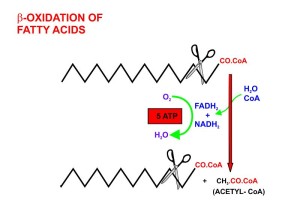 The many cycles whereby food is metabolized into small molecules are also signals for the clock cycles. Many of these are connected to daily rhythms. Polyamines are small molecules that regulate, through complex cycles, many of the interactions of proteins with proteins, and proteins with DNA.
The many cycles whereby food is metabolized into small molecules are also signals for the clock cycles. Many of these are connected to daily rhythms. Polyamines are small molecules that regulate, through complex cycles, many of the interactions of proteins with proteins, and proteins with DNA.
One energy molecule is particularly important for the clock cycles—NAD+ (+ means it is oxidized). It can carry electrons to transfer in chemical reactions. It can alter protein activity. It part of a vast number of very complex cycles, loops, and pathways.
Another important molecule is Acetyl CoA, which is a primary energy molecule and a major part of basic processes as glycolysis and metabolism of amino acids in mitochondria. Acetyl CoA is highly connected to the many clock mechanisms in the cytoplasm and can go into the nucleus where it affects enzymes in rhythmic patterns. Acetyl CoA rhythms are vital for production of fatty acids, cholesterol, bile acids, steroids, and ketone bodies and many of these cycles are connected to the clock. In mitochondria they are part of the TCA cycle.
Cholesterol, made from acetyl CoA, is highly regulated by the clock. Another very complex pathway controls bile acids with increase in acids and decrease in cholesterol. Bile acids in the blood then influences brown fat affecting temperature regulation. Temperature has many effects throughout the body that are also complex cycles in different organs connected daily rhythms. This also interacts with melatonin production.
Brain Integration of Metabolic Information
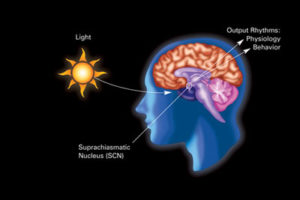 Signals that are sent into the blood from these metabolic pathways and cycles go back to the brain and influence the brain’s clocks including the SCN. Some of these signals cannot override the strong direction of the SCN based on light cycles. Feeding rhythms are often too strong also for the centers in the pituitary, hypothalamus, and PVN. The triggers, pathways, and relationships among these brain centers are not understood and relate to energy balance, feeding and fasting, and light and dark, among other variables. Also temperature and activity also interact with all of these.
Signals that are sent into the blood from these metabolic pathways and cycles go back to the brain and influence the brain’s clocks including the SCN. Some of these signals cannot override the strong direction of the SCN based on light cycles. Feeding rhythms are often too strong also for the centers in the pituitary, hypothalamus, and PVN. The triggers, pathways, and relationships among these brain centers are not understood and relate to energy balance, feeding and fasting, and light and dark, among other variables. Also temperature and activity also interact with all of these.
Perhaps the most researched variable is a special diet called timed restrictive feeding or TRF. This has interactions with energy balance cycles including absorption, storage of the molecules, and use of the material. Another variable just now being un covered are the effects of microbes.
There is now no doubt that the schedule of life activities, particularly eating and sleeping, have major impact on whether we will develop multiple different diseases.
Cellular Clocks and Metabolism
 The central clocks send out rhythmic signals that synchronize many cell functions across the body tissues. But individual cells each have their own clock as well as many very specific loops and cycles related to the particular functions in the tissues. Somehow all of this is coordinated the level of the brain, the bodily organs, and the individual cells. Along with cells knowing how big they should be, where they should be, and their functions in the tissue, communication among all cells is ubiquitous and extremely complex.
The central clocks send out rhythmic signals that synchronize many cell functions across the body tissues. But individual cells each have their own clock as well as many very specific loops and cycles related to the particular functions in the tissues. Somehow all of this is coordinated the level of the brain, the bodily organs, and the individual cells. Along with cells knowing how big they should be, where they should be, and their functions in the tissue, communication among all cells is ubiquitous and extremely complex.
The next post is about cycles in immune function.