 For many years, amyloid toxic plaque accumulation has been considered to be the way brains are damaged in Alzheimer’s disease. Almost all experimental treatments have tried to stop this accumulation and none have helped. In recent years, the association of abnormal deposits of the vital tau protein that holds microtubules together and brain damage of Alzheimer’s has become more evident.
For many years, amyloid toxic plaque accumulation has been considered to be the way brains are damaged in Alzheimer’s disease. Almost all experimental treatments have tried to stop this accumulation and none have helped. In recent years, the association of abnormal deposits of the vital tau protein that holds microtubules together and brain damage of Alzheimer’s has become more evident.
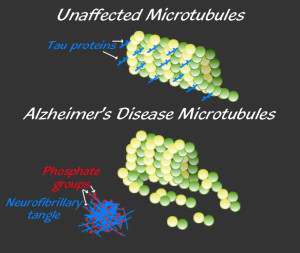
The most up to date research is showing a connection of early amyloid changes somehow related to late tau changes that might be the most damaging. The abnormal tau is not well understood despite a large amount of research. But, it is thought that multiple abnormal types appear related to abnormal tagging of the tau molecule with phosphates that destabilizes the very important microtubules in the brain. (see posts that show how vital these highways are in the neuron for brain function). A previous post showed that tau is involved in many other brain diseases as well—called tauopathies. All of these have abnormal attachment of these phosphates—the process called phosphorylation uses the most prevalent enzymes in the body called kinases.
Now, the most recent research adds more complexity. It shows that there are a large amount of different ways that tau is tagged with phosphate and that some specific types of phosphate tagging are in fact protective to the brain. This implies that there is an active process that tags and untags tau as part of normal functions. Also, recent research shows that tau is also involved in the scaffolding in the nucleus for DNA functions. This post will summarize the previous posts that on tau research below after the new data on how some tagging of tau is protective.
Tau Tagging in Alzheimer’s and Protection Against Alzheimer’s
When the tau molecule is phosphorylated it can become a toxic molecule that forms clumps and then neurofibrillary tangles—the second hallmark of Alzheimer’s with the first being amyloid plaques. In studies of brains of those with Alzheimer’s, tau has a large amount of phosphate tags, called hyper phosphorylation. These are highly correlated with the death of neurons, the cause of the memory loss and other symptoms in dementia.
However, research last year showed tagging on a specific amino acid of the tau—threonine 205—helped avoid the damage from amyloid toxicity. This was opposite of what most thought could occur. Other research in mice showed that damage could be lessened when tau is genetically eliminated. This ability to protect the neuron has been termed pT2015 tau-associated neuroprotection.
The exact mechanism of this protection is only now being evaluated. A particular kinase enzyme (kinases place phosphates on tau) is p38. It was thought to be involved in this protection and research has included four variants—p38a, p38b, p38g, and p38d. In some experiments one particular variant—p38g—is correlated with less neuronal excitability and induced seizures. Mice also had alterations in cognitive ability without this particular variant and there were less seizures. When there was an increase of tau in general, but no p38g, there was increase in seizures in the mice. The other variants did not protect against seizures from neuronal damage. These experiments show that this variant, not the other types, regulate the kind of toxicity from amyloid destruction that causes excitatory phenomena such as seizures from the toxicity.
 New research shows a correlation with this variant and the complex in the post synaptic membrane. At the synapse very specialized mechanisms are used for neurotransmitters to be sent from one neuron and to trigger the next. There are special mechanisms to prepare for the rapid sequential secretion of vesicles with neurotransmitters. There is also a very specialized large complex mechanism called the post synaptic density—PSD 95.
New research shows a correlation with this variant and the complex in the post synaptic membrane. At the synapse very specialized mechanisms are used for neurotransmitters to be sent from one neuron and to trigger the next. There are special mechanisms to prepare for the rapid sequential secretion of vesicles with neurotransmitters. There is also a very specialized large complex mechanism called the post synaptic density—PSD 95.
Tau in this variant triggers actions in the PSD and a special kinase is related to this—kinase fyn. This kinase previously increased seizures in other experiments. Another piece of this puzzle is part of the receptor for NMDA, which is the most prominent glutamate receptor in the brain related to learning and memory. It appears that the variant of tau promotes disconnect of the PSD related to the hyper excitability.
Further research attempted to find how this kinase variant produces the effect—that is, where the phosphate tag goes and how this affects learning and seizures in mice. One site called T205 on tau is affected. This site is where fyn takes a phosphate at T205 or it alter s it. There are more seizures without the tagging and less when it is tagged.
Tau has many different places where these phosphate tags are attached and many of these could regulate functions with tagging and un tagging. Tagging and un tagging occurs at Ser214, Thr231 and Ser262 at least in order to attach to microtubules in good neurons along the axon highways. Interactions of these affect the PSD as well such as Ser212/Thr214, Ser202/Thr205, and Thr231 by altering the tau interaction with the PSD.
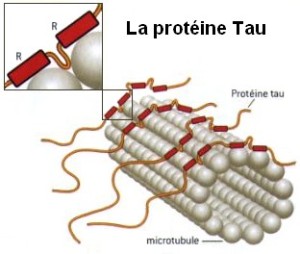
It appears that these particular sites use the process in complex functions for normal neurons. This changes the view of phosphorylation of tau into a normal function that is regulated and makes evaluation of its effect in dementia much more complex.
This research is viewed in the context of experiences where genetic elimination of tau protects from this toxicity from amyloid clumps. Lowering the total amount helps. But, elimination of all tagged tau goes against this new research where a particular variant might be essential for regulation of the normal functions. Some experiments show that eliminating some variants are negative. Now, it is possible that the targets of treatments could be at the specific tags such as phosphorylation of T205. These targeting effects on tags might even be reversible and maintain specific functions.
The new research makes it all more complex because the particular type of tau and exactly where it is tagged appears to be vital for both the toxicity, for protection from toxicity, and for normal functions. This also strongly implies that the processes of the two enzyme systems of tagging and un tagging phosphates on tau are part of normal function as they are in histones and DNA.
Summary of Previous Posts
The argument for tau causing Alzheimer’s:
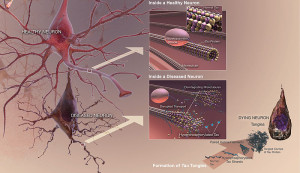 Recent research has shown that many (possibly 30%) of Alzheimer patients have no amyloid buildup and many normal elders do have amyloid in the brain (See post on Amyloid and Alzheimer’s). Therefore, amyloid as a cause of Alzheimer’s is not at all certain.
Recent research has shown that many (possibly 30%) of Alzheimer patients have no amyloid buildup and many normal elders do have amyloid in the brain (See post on Amyloid and Alzheimer’s). Therefore, amyloid as a cause of Alzheimer’s is not at all certain.
Tau is a critical protein that holds together the very active microtubules that build and rebuild structures in the axon and dendrite (see post on axon transport). A very complex relationship of tau, amyloid-β and inflammation is emerging as possible causes of Alzheimer’s. Tau, itself, appears to be correlated with many other forms of brain disease. But, the clumps don’t exactly correlate with Alzheimer’s, but the transmission of tau (of some unknown type) along specific neuronal circuits does. In fact, these circuits map out the exact development of Alzheimer’s in the brain. One problem of animal research with tau is that mice only produce half of the 6 types of tau. Also, they are different in the N-terminal header section.
 It is not known what the travelling destructive toxic tau consists of: large aggregates, small oligomers), or abnormal tau molecules that are soluble in water.
It is not known what the travelling destructive toxic tau consists of: large aggregates, small oligomers), or abnormal tau molecules that are soluble in water.
There are many different kinds of abnormal tau and many different brain diseases are related to these. Many are from a large number of different tags on tau, similar to epigenetic tags on DNA and histones. Both of these require enzymes to place and remove the tags.
Tau Basics
 Tau is an unusual protein in that it is often unfolded, unlike most proteins that have highly specific shapes determining their functions. This may allow it to have special and multiple functions. Tau is critical for stabilizing microtubules in the neuron, which have been described in previous posts as the LEGO brain of the neuron, constantly building and rebuilding axons and dendrites in response to thought. Normal tau is soluble in water and doesn’t naturally combine with other tau molecules to form aggregates.
Tau is an unusual protein in that it is often unfolded, unlike most proteins that have highly specific shapes determining their functions. This may allow it to have special and multiple functions. Tau is critical for stabilizing microtubules in the neuron, which have been described in previous posts as the LEGO brain of the neuron, constantly building and rebuilding axons and dendrites in response to thought. Normal tau is soluble in water and doesn’t naturally combine with other tau molecules to form aggregates.
Tau is called a “microtubule associated protein”, one of many stabilizing and regulating the vast amount of shapes that microtubules form. The gene that makes tau is called MAPT for microtubule-associated protein tau gene.
Tau is also important in the nucleus. It binds to RNA by electro chemical interactions and it may also help stabilize RNA and DNA structures in the nucleus, as well as microtubules. It is possible that this interaction with RNA is part of the problem that forms aggregates of abnormal tau. One of the tau variants (4R) is better at helping microtubules.
One particular region of the tau protein that faces away from the attachment to microtubules is called the projection domain. This section of tau is influential in determining the space between two microtubules that are stabilized by tau. Different versions of this N-terminal of the amino acid affects where the different tau are located inside the cell and, also, are related to which will form tangles.
Normal Tau Functions
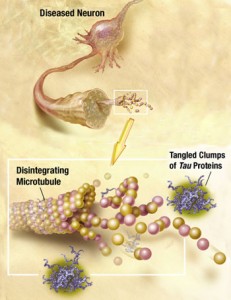 The major function of tau is thought to be stabilizing microtubules by regulating the instability of the microtubule structures. This allows the cytoskeleton to rapidly change structures in the axon and dendrite. There are only small areas of the tau molecule that actually bind to the α and β-tubulins of the microtubule. The rest of the molecule remains flexible and changeable for new shapes. In fact, the normal regions, which become part of abnormal clumps, usually form a hairpin structure that looks like a paper clip.
The major function of tau is thought to be stabilizing microtubules by regulating the instability of the microtubule structures. This allows the cytoskeleton to rapidly change structures in the axon and dendrite. There are only small areas of the tau molecule that actually bind to the α and β-tubulins of the microtubule. The rest of the molecule remains flexible and changeable for new shapes. In fact, the normal regions, which become part of abnormal clumps, usually form a hairpin structure that looks like a paper clip.
Tau, also, regulates transport along the microtubules, influencing dynein and kinesin motors’ movement in both directions toward the cell body and toward the terminal end of the axon at the synapse. It competes with these motors regulating their speeds and movements. It slows them down for pickups, such as mitochondria.
Tau is a cargo itself and competes with other types of transport. Tau regulates release of vesicle cargoes. It interacts especially with dyactin a cofactor of dynein motor. But, the real influences are not fully understood. Tau is needed for axons to grow. (See post on transport along the axon).
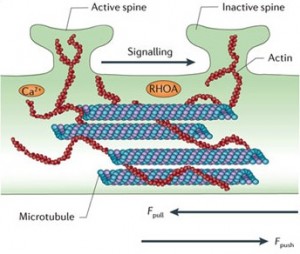 Recent research demonstrates a definite effect on neuroplasticity and long-term potentiation, but the mechanisms are not yet clear. It, also, interacts with the proteasome, a structure that grinds up used proteins to recycle the material.
Recent research demonstrates a definite effect on neuroplasticity and long-term potentiation, but the mechanisms are not yet clear. It, also, interacts with the proteasome, a structure that grinds up used proteins to recycle the material.
Tau is important in the nucleus of neurons and fibroblasts and in neuronal stem cells to help the scaffolding of the complex structures of DNA (see post), the positions of RNA in the nucleus, and the cytoplasm. More is being learned about how the exact locations of chromatin in the nucleus are, in fact, critical to which parts of DNA are utilized. Tau is involved in stabilizing and regulating this internal three dimensional nuclear structure.
Tau is involved in regulating the activity of neurons during migration. It is involved in the transport of iron in the neuron. Tau, also, interacts with the actin structures, which form many of the other structures in the changing neuron.
Tau lives mostly inside cells, but is secreted from neurons under some conditions. The more electrical and synapse activity in the neuron, the more tau is released outside the cell.
Adding of Phosphate to Tau
 In the fetus, tau has seven phosphates attached (phosphorylation), whereas adults only have two.In Alzheimer’s, the number of attached phosphorus particles increases to eight, at least. There might, in fact be more phosphates, but most studies are from cadavers, which could have lost some of them through enzyme actions.
In the fetus, tau has seven phosphates attached (phosphorylation), whereas adults only have two.In Alzheimer’s, the number of attached phosphorus particles increases to eight, at least. There might, in fact be more phosphates, but most studies are from cadavers, which could have lost some of them through enzyme actions.
There are 85 places where phosphates can attach and 45 have been seen in research. Two sites seem to be related to the brain diseases (17 Thr-Pro or Ser-Pro) and are related to enzymes that work with serine, proline and threonine amino acids. The type of enzyme that places high-energy phosphorus particles on molecules is called a kinase. Kinases are one of the largest families of proteins in all of nature. There are many different kinases involved in this process and there are variations in what section is phosphorylated in Alzheimer’s.
An opposite type of enzyme to the kinase takes phosphorus tags off of molecules and there are many of these phosphatases, also. One in particular (PP2A) does 70% of this activity in the human brain and there is less with Alzheimer’s (20% less in the grey matter or cell bodies and 40% less in the white matter or axons.)
Previously it was found phosphorylation is a critical part of normal regulation of tau activity and influences microtubule assembly and stabilization. Adding more phosphate to one region in tau changes its attachment to microtubules. Another regional change causes release of tau from the microtubule. Now, some variant of the tagging is protective against brain disease.
More Complexity with Tau
 As with everything else in the brain, the story of tau in healthy brains and disease is extraordinarily complex. There is clearly some relationship with amyloid–β, but it is not clear. It is related to inflammation, but this is not clear, either. It functions as a stabilizing agent in the microtubules of the axon highway and in nucleus with DNA and RNA. Some tagged version of tau seems to be the culprit in Alzheimer’s, but it is not clear which.
As with everything else in the brain, the story of tau in healthy brains and disease is extraordinarily complex. There is clearly some relationship with amyloid–β, but it is not clear. It is related to inflammation, but this is not clear, either. It functions as a stabilizing agent in the microtubules of the axon highway and in nucleus with DNA and RNA. Some tagged version of tau seems to be the culprit in Alzheimer’s, but it is not clear which.
Now, research shows that tagging tau with phosphates are vital for protection of brain disease. The tagging of tau un tagging are becoming more complex as with the histones and DNA in the nucleus. It is involved in the microtubule process as well as causing and stopping brain disease. This now appears to be a normal process that is regulated by two opposing sets of enzymes and is vastly complex as is everything else in the brain.